当前位置:
X-MOL 学术
›
Transl. Psychiaty
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
1,25-Dihydroxyvitamin D modulates L-type voltage-gated calcium channels in a subset of neurons in the developing mouse prefrontal cortex.
Translational Psychiatry ( IF 5.8 ) Pub Date : 2019-11-11 , DOI: 10.1038/s41398-019-0626-z Helen Gooch 1 , Xiaoying Cui 1 , Victor Anggono 1, 2 , Maciej Trzaskowski 3 , Men Chee Tan 1, 2 , Darryl W Eyles 1, 4 , Thomas H J Burne 1, 4 , Se Eun Jang 1, 2 , Manuel Mattheisen 5, 6 , David M Hougaard 6, 7 , Bent Nørgaard Pedersen 7 , Arieh Cohen 7 , Preben B Mortensen 6, 8, 9 , Pankaj Sah 1, 10 , John J McGrath 1, 4, 8
Translational Psychiatry ( IF 5.8 ) Pub Date : 2019-11-11 , DOI: 10.1038/s41398-019-0626-z Helen Gooch 1 , Xiaoying Cui 1 , Victor Anggono 1, 2 , Maciej Trzaskowski 3 , Men Chee Tan 1, 2 , Darryl W Eyles 1, 4 , Thomas H J Burne 1, 4 , Se Eun Jang 1, 2 , Manuel Mattheisen 5, 6 , David M Hougaard 6, 7 , Bent Nørgaard Pedersen 7 , Arieh Cohen 7 , Preben B Mortensen 6, 8, 9 , Pankaj Sah 1, 10 , John J McGrath 1, 4, 8
Affiliation
|
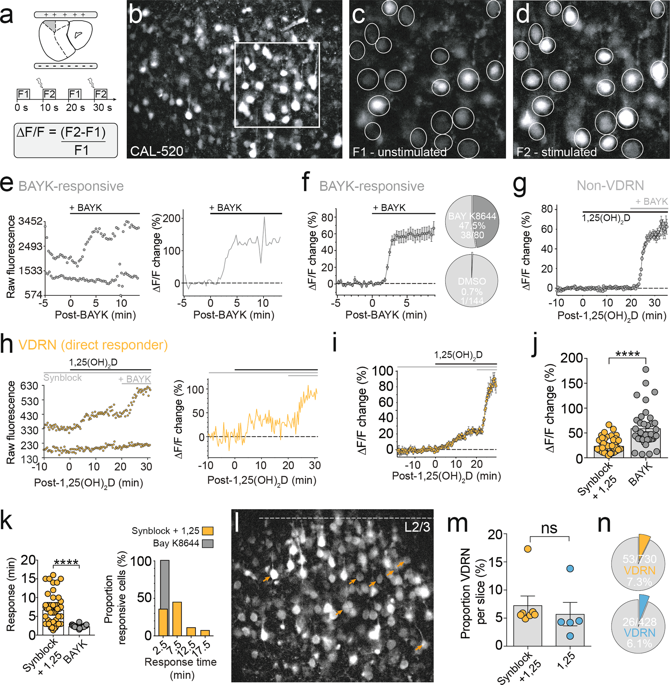
Schizophrenia has been associated with a range of genetic and environmental risk factors. Here we explored a link between two risk factors that converge on a shared neurobiological pathway. Recent genome-wide association studies (GWAS) have identified risk variants in genes that code for L-type voltage-gated calcium channels (L-VGCCs), while epidemiological studies have found an increased risk of schizophrenia in those with neonatal vitamin D deficiency. The active form of vitamin D (1,25(OH)2D) is a secosteroid that rapidly modulates L-VGCCs via non-genomic mechanisms in a range of peripheral tissues, though its non-genomic effects within the brain remain largely unexplored. Here we used calcium imaging, electrophysiology and molecular biology to determine whether 1,25(OH)2D non-genomically modulated L-VGCCs in the developing prefrontal cortex, a region widely implicated in schizophrenia pathophysiology. Wide-field Ca2+ imaging revealed that physiological concentrations of 1,25(OH)2D rapidly enhanced activity-dependent somatic Ca2+ levels in a small subset of neurons in the developing PFC, termed vitamin D-responsive neurons (VDRNs). Somatic nucleated patch recordings revealed a rapid, 1,25(OH)2D-evoked increase in high-voltage-activated (HVA) Ca2+ currents. Enhanced activity-dependent Ca2+ levels were mediated by L-VGCC but not associated with any changes to Cacna1c (L-VGCC pore-forming subunit) mRNA expression. Since L-VGCC activity is critical to healthy neurodevelopment, these data suggest that suboptimal concentrations of 1,25(OH)2D could alter brain maturation through modulation of L-VGCC signalling and as such may provide a parsimonious link between epidemiologic and genetic risk factors for schizophrenia.
中文翻译:

1,25-二羟基维生素D调节发育中的小鼠前额叶皮层神经元子集中的L型电压门控钙通道。
精神分裂症与一系列遗传和环境危险因素有关。在这里,我们探讨了在共同的神经生物学途径中收敛的两个风险因素之间的联系。最近的全基因组关联研究(GWAS)已确定了编码L型电压门控钙通道(L-VGCC)的基因中的风险变异,而流行病学研究发现新生儿维生素D缺乏者的精神分裂症风险增加。维生素D(1,25(OH)2D)的活性形式是一种类固醇,它通过非基因组机制在周围组织中快速调节L-VGCC,尽管在大脑中的非基因组作用仍未得到充分探索。在这里,我们使用钙成像,电生理学和分子生物学方法来确定发育中的前额叶皮层中是否存在1,25(OH)2D非基因组调节的L-VGCC,广泛参与精神分裂症病理生理的区域。宽视野Ca2 +成像显示,生理浓度的1,25(OH)2D在发育中的PFC的一小部分神经元(称为维生素D反应性神经元(VDRNs))中迅速增强了依赖于活性的体细胞Ca2 +水平。体细胞核化的补丁记录显示,高压激活(HVA)Ca2 +电流在1,25(OH)2D诱发下迅速增加。增强的依赖于活性的Ca2 +水平由L-VGCC介导,但与Cacna1c(L-VGCC孔形成亚基)mRNA表达的任何变化都不相关。由于L-VGCC活性对于健康的神经发育至关重要,因此这些数据表明,次最佳浓度为1,
更新日期:2019-11-11
中文翻译:

1,25-二羟基维生素D调节发育中的小鼠前额叶皮层神经元子集中的L型电压门控钙通道。
精神分裂症与一系列遗传和环境危险因素有关。在这里,我们探讨了在共同的神经生物学途径中收敛的两个风险因素之间的联系。最近的全基因组关联研究(GWAS)已确定了编码L型电压门控钙通道(L-VGCC)的基因中的风险变异,而流行病学研究发现新生儿维生素D缺乏者的精神分裂症风险增加。维生素D(1,25(OH)2D)的活性形式是一种类固醇,它通过非基因组机制在周围组织中快速调节L-VGCC,尽管在大脑中的非基因组作用仍未得到充分探索。在这里,我们使用钙成像,电生理学和分子生物学方法来确定发育中的前额叶皮层中是否存在1,25(OH)2D非基因组调节的L-VGCC,广泛参与精神分裂症病理生理的区域。宽视野Ca2 +成像显示,生理浓度的1,25(OH)2D在发育中的PFC的一小部分神经元(称为维生素D反应性神经元(VDRNs))中迅速增强了依赖于活性的体细胞Ca2 +水平。体细胞核化的补丁记录显示,高压激活(HVA)Ca2 +电流在1,25(OH)2D诱发下迅速增加。增强的依赖于活性的Ca2 +水平由L-VGCC介导,但与Cacna1c(L-VGCC孔形成亚基)mRNA表达的任何变化都不相关。由于L-VGCC活性对于健康的神经发育至关重要,因此这些数据表明,次最佳浓度为1,











































 京公网安备 11010802027423号
京公网安备 11010802027423号