当前位置:
X-MOL 学术
›
Thermochim. Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermodynamic functions of holmium orthophosphate HoPO4 in the range 9−1370 K
Thermochimica Acta ( IF 3.1 ) Pub Date : 2020-01-01 , DOI: 10.1016/j.tca.2019.178459 A.V. Tyurin , M.A. Ryumin , A.V. Khoroshilov , V.M. Gurevich , K.S. Gavrichev
Thermochimica Acta ( IF 3.1 ) Pub Date : 2020-01-01 , DOI: 10.1016/j.tca.2019.178459 A.V. Tyurin , M.A. Ryumin , A.V. Khoroshilov , V.M. Gurevich , K.S. Gavrichev
|
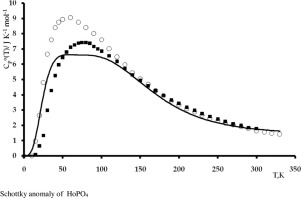
Abstract The heat capacity of holmium orthophosphate HoPO4 was studied using an adiabatic and a differential scanning calorimetry in the temperatures intervals 9–338 and 310–1374 K, relatively. Heat capacity anomaly connected with the splitting of ground state electronic levels of f-shell was determined. Values of smoothed thermodynamic functions (heat capacity, entropy, enthalpy change and derived Gibbs energy) in the wide temperature range 9–1374 K were calculated.
中文翻译:

正磷酸钬 HoPO4 在 9−1370 K 范围内的热力学函数
摘要 使用绝热和差示扫描量热法在 9-338 和 310-1374 K 的温度区间内相对地研究了正磷酸钬 HoPO4 的热容量。确定了与 f 壳基态电子能级分裂相关的热容异常。计算了 9-1374 K 宽温度范围内的平滑热力学函数值(热容、熵、焓变和派生吉布斯能)。
更新日期:2020-01-01
中文翻译:

正磷酸钬 HoPO4 在 9−1370 K 范围内的热力学函数
摘要 使用绝热和差示扫描量热法在 9-338 和 310-1374 K 的温度区间内相对地研究了正磷酸钬 HoPO4 的热容量。确定了与 f 壳基态电子能级分裂相关的热容异常。计算了 9-1374 K 宽温度范围内的平滑热力学函数值(热容、熵、焓变和派生吉布斯能)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号