Phytochemistry Letters ( IF 1.3 ) Pub Date : 2019-11-11 , DOI: 10.1016/j.phytol.2019.10.014 Marie Schmitt , Abdulmagid Alabdul Magid , Jane Hubert , Nicolas Etique , Laurent Duca , Laurence Voutquenne-Nazabadioko
|
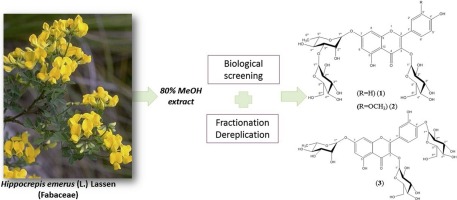
This study presents the bio-guided chemical investigation of a 80% methanol extract of Hippocrepis emerus flowers, a perennial non-climbing shrub. Liquid-liquid partitioning in solvents of increasing polarity combined to biological screening enabled to determine the EtOAc and n-BuOH soluble fractions as the most active parts of the extract. These fractions were chemically profiled by using a 13C NMR-based dereplication method, resulting in the identification of twenty-six compounds. The dereplication process was completed by purification of some unknown or minor compounds of the n-BuOH fraction. Three new glycosylated flavonoids, namely kaempferol-3-O-β-d-glucopyranosyl-7-O-β-d-glucopyranosyl-(1→3)-α-l-rhamnopyranoside (1), isorhamnetin-3-O-β-d-glucopyranosyl-7-O-β-d-glucopyranosyl-(1→3)-α-l-rhamnopyranoside (2) and quercetin-3-O-β-d-glucopyranosyl-7,4′-O-α-l-dirhamnopyranoside (3), together with twelve known compounds (4 – 15) were isolated. Their structures were elucidated by spectroscopic methods including NMR, HR-ESI-MS and UV. The antioxidant activity of fractions and isolated compounds were evaluated using DPPH and hydroxyl radical scavenging and CUPRAC assays. In parallel, their inhibitory properties against mushroom tyrosinase and human neutrophil elastase enzymes were assessed. Three quercetin glycosides exhibited a significant radical-scavenging activity and two flavonoids showed a moderate elastase inhibitory activity.
中文翻译:

生物引导下的紫花海草花中新酚类化合物的分离及其抗氧化,酪氨酸酶和弹性蛋白酶抑制活性的研究
这项研究提出了一种生物引导的化学研究方法,该方法是对多年生非爬坡灌木紫花沙棘花的80%甲醇提取物进行的研究。极性增加的溶剂中的液-液分配与生物筛选相结合,能够确定EtOAc和n- BuOH可溶性馏分作为提取物中最活泼的部分。通过使用基于13 C NMR的重复复制法对这些馏分进行化学分析,从而鉴定出26种化合物。去复制过程通过纯化一些未知的或少量的n- BuOH部分化合物来完成。三种新的糖基化类黄酮,即kaempferol-3- O - β - d-吡喃葡萄糖基-7-ø -β- d -glucopyranosyl-(1→3)-α-升-rhamnopyranoside(1),异鼠李素-3- ö -β- d吡喃葡萄糖基-7- ö -β- d -glucopyranosyl-(1→3) -α-升-rhamnopyranoside(2)和槲皮素-3- ö -β- d吡喃葡萄糖基- 7,4'- ö -α-升-dirhamnopyranoside(3具有十二个已知化合物(),一起4 - 15)被隔离。通过包括NMR,HR-ESI-MS和UV在内的光谱方法阐明了它们的结构。使用DPPH和清除羟基自由基和CUPRAC分析评估馏分和分离的化合物的抗氧化活性。同时,评估了它们对蘑菇酪氨酸酶和人嗜中性粒细胞弹性蛋白酶的抑制特性。三种槲皮素糖苷具有明显的自由基清除活性,两种黄酮类化合物具有中等弹性蛋白酶抑制活性。









































 京公网安备 11010802027423号
京公网安备 11010802027423号