Metabolic Engineering ( IF 6.8 ) Pub Date : 2019-11-09 , DOI: 10.1016/j.ymben.2019.11.004 Kentaro Inokuma 1 , Hiroki Kurono 1 , Riaan den Haan 2 , Willem Heber van Zyl 3 , Tomohisa Hasunuma 4 , Akihiko Kondo 5
|
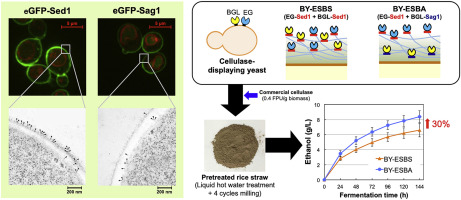
The yeast cell surface provides space to display functional proteins. Heterologous proteins can be covalently anchored to the yeast cell wall by fusing them with the anchoring domain of glycosylphosphatidylinositol (GPI)-anchored cell wall proteins (GPI-CWPs). In the yeast cell-surface display system, the anchorage position of the target protein in the cell wall is an important factor that maximizes the capabilities of engineered yeast cells because the yeast cell wall consists of a 100- to 200-nm-thick microfibrillar array of glucan chains. However, knowledge is limited regarding the anchorage position of GPI-attached proteins in the yeast cell wall. Here, we report a comparative study on the effect of GPI-anchoring domain–heterologous protein fusions on yeast cell wall localization. GPI-anchoring domains derived from well-characterized GPI-CWPs, namely Sed1p and Sag1p, were used for the cell-surface display of heterologous proteins in the yeast Saccharomyces cerevisiae. Immunoelectron-microscopic analysis of enhanced green fluorescent protein (eGFP)-displaying cells revealed that the anchorage position of the GPI-attached protein in the cell wall could be controlled by changing the fused anchoring domain. eGFP fused with the Sed1-anchoring domain predominantly localized to the external surface of the cell wall, whereas the anchorage position of eGFP fused with the Sag1-anchoring domain was mainly inside the cell wall. We also demonstrate the application of the anchorage position control technique to improve the cellulolytic ability of cellulase-displaying yeast. The ethanol titer during the simultaneous saccharification and fermentation of hydrothermally-processed rice straw was improved by 30% after repositioning the exo- and endo-cellulases using Sed1- and Sag1-anchor domains. This novel anchorage position control strategy will enable the efficient utilization of the cell wall space in various fields of yeast cell-surface display technology.
中文翻译:

使用不同的GPI锚定域控制酵母细胞壁中GPI附着蛋白的锚定位置控制的新策略。
酵母细胞表面提供了展示功能蛋白的空间。异源蛋白可以通过将它们与糖基磷脂酰肌醇(GPI)锚定的细胞壁蛋白(GPI-CWPs)的锚定域融合而共价锚定到酵母细胞壁上。在酵母细胞表面展示系统中,靶蛋白在细胞壁中的锚定位置是最大化工程酵母细胞功能的重要因素,因为酵母细胞壁由100至200 nm厚的微纤维阵列组成葡聚糖链。然而,关于GPI附着蛋白在酵母细胞壁中的锚定位置的知识是有限的。在这里,我们报告了GPI锚定域-异源蛋白融合对酵母细胞壁定位的影响的比较研究。GPI锚定域源自特征明确的GPI-CWP,酿酒酵母。增强型绿色荧光蛋白(eGFP)展示细胞的免疫电子显微镜分析表明,可以通过改变融合的锚定域来控制GPI附着蛋白在细胞壁中的锚定位置。与Sed1锚定域融合的eGFP主要位于细胞壁的外表面,而与Sag1锚定域融合的eGFP的锚定位置主要在细胞壁内部。我们还证明了锚定位置控制技术在提高纤维素酶展示酵母的纤维素分解能力方面的应用。使用Sed1和Sag1锚结构域重新定位胞外和内切纤维素酶后,水热处理的稻草同时糖化和发酵过程中的乙醇滴度提高了30%。











































 京公网安备 11010802027423号
京公网安备 11010802027423号