当前位置:
X-MOL 学术
›
BBA Bioenerg.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Integrated thermodynamic analysis of electron bifurcating [FeFe]-hydrogenase to inform anaerobic metabolism and H2 production.
Biochimica et Biophysica Acta (BBA) - Bioenergetics ( IF 3.4 ) Pub Date : 2019-11-08 , DOI: 10.1016/j.bbabio.2019.148087 Zackary J Jay 1 , Kristopher A Hunt 1 , Katherine J Chou 2 , Gerrit J Schut 3 , Pin-Ching Maness 2 , Michael W W Adams 3 , Ross P Carlson 1
Biochimica et Biophysica Acta (BBA) - Bioenergetics ( IF 3.4 ) Pub Date : 2019-11-08 , DOI: 10.1016/j.bbabio.2019.148087 Zackary J Jay 1 , Kristopher A Hunt 1 , Katherine J Chou 2 , Gerrit J Schut 3 , Pin-Ching Maness 2 , Michael W W Adams 3 , Ross P Carlson 1
Affiliation
|
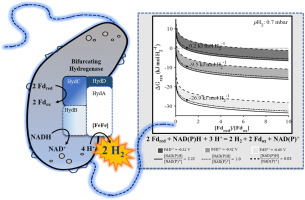
Electron bifurcating, [FeFe]-hydrogenases are recently described members of the hydrogenase family and catalyze a combination of exergonic and endergonic electron exchanges between three carriers (2 ferredoxinred- + NAD(P)H + 3 H+ = 2 ferredoxinox + NAD(P)+ + 2 H2). A thermodynamic analysis of the bifurcating, [FeFe]-hydrogenase reaction, using electron path-independent variables, quantified potential biological roles of the reaction without requiring enzyme details. The bifurcating [FeFe]-hydrogenase reaction, like all bifurcating reactions, can be written as a sum of two non-bifurcating reactions. Therefore, the thermodynamic properties of the bifurcating reaction can never exceed the properties of the individual, non-bifurcating, reactions. The bifurcating [FeFe]-hydrogenase reaction has three competitive properties: 1) enabling NAD(P)H-driven proton reduction at pH2 higher than the concurrent operation of the two, non-bifurcating reactions, 2) oxidation of NAD(P)H and ferredoxin simultaneously in a 1:1 ratio, both are produced during typical glucose fermentations, and 3) enhanced energy conservation (~10 kJ mol-1 H2) relative to concurrent operation of the two, non-bifurcating reactions. Our analysis demonstrated ferredoxin E°' largely determines the sensitivity of the bifurcating reaction to pH2, modulation of the reduced/oxidized electron carrier ratios contributed less to equilibria shifts. Hydrogenase thermodynamics data were integrated with typical and non-typical glycolysis pathways to evaluate achieving the 'Thauer limit' (4 H2 per glucose) as a function of temperature and pH2. For instance, the bifurcating [FeFe]-hydrogenase reaction permits the Thauer limit at 60 °C if pH 2 ≤ ~10 mbar. The results also predict Archaea, expressing a non-typical glycolysis pathway, would not benefit from a bifurcating [FeFe]-hydrogenase reaction; interestingly, no Archaea have been observed experimentally with a [FeFe]-hydrogenase enzyme.
中文翻译:

电子分叉[FeFe]-加氢酶的综合热力学分析,以告知厌氧代谢和氢气产生。
电子分叉[FeFe]-加氢酶最近被描述为加氢酶家族的成员,并催化三种载流子之间的强电子和负电子交换的组合(2个铁氧还蛋白-+ NAD(P)H + 3 H + = 2铁氧还蛋白+ NAD(P) + + 2 H2)。使用不依赖电子路径的变量对分叉的[FeFe]-加氢酶反应进行热力学分析,无需酶的详细信息即可定量反应的潜在生物学作用。像所有分叉反应一样,分叉的[FeFe]-加氢酶反应可以写成两个非分叉反应的总和。因此,分叉反应的热力学性质决不能超过单个非分叉反应的性质。分叉的[FeFe]-加氢酶反应具有三个竞争特性:1)在pH2下能使NAD(P)H驱动的质子还原率高于两个非分叉反应的同时操作; 2)同时产生NAD(P)H和铁氧还蛋白以1:1的比例氧化在典型的葡萄糖发酵过程中,以及3)相对于两个非分叉反应的同时运行,提高了能量守恒(〜10 kJ mol-1 H2)。我们的分析表明,铁氧还蛋白E'在很大程度上决定了分叉反应对pH2的敏感性,对还原/氧化电子载流子比例的调节对平衡移动的贡献较小。将氢化酶热力学数据与典型的和非典型的糖酵解途径整合在一起,以评估达到的“ Thauer极限”(每个葡萄糖4 H2)与温度和pH2的关系。例如,如果pH 2≤〜10 mbar,则分叉的[FeFe]-加氢酶反应允许在60°C下的Tauer极限。该结果还预测,表达非典型糖酵解途径的古生菌将不会从分叉的[FeFe]-氢化酶反应中受益。有趣的是,在实验中没有用[FeFe]氢化酶观察到古细菌。
更新日期:2019-11-11
中文翻译:

电子分叉[FeFe]-加氢酶的综合热力学分析,以告知厌氧代谢和氢气产生。
电子分叉[FeFe]-加氢酶最近被描述为加氢酶家族的成员,并催化三种载流子之间的强电子和负电子交换的组合(2个铁氧还蛋白-+ NAD(P)H + 3 H + = 2铁氧还蛋白+ NAD(P) + + 2 H2)。使用不依赖电子路径的变量对分叉的[FeFe]-加氢酶反应进行热力学分析,无需酶的详细信息即可定量反应的潜在生物学作用。像所有分叉反应一样,分叉的[FeFe]-加氢酶反应可以写成两个非分叉反应的总和。因此,分叉反应的热力学性质决不能超过单个非分叉反应的性质。分叉的[FeFe]-加氢酶反应具有三个竞争特性:1)在pH2下能使NAD(P)H驱动的质子还原率高于两个非分叉反应的同时操作; 2)同时产生NAD(P)H和铁氧还蛋白以1:1的比例氧化在典型的葡萄糖发酵过程中,以及3)相对于两个非分叉反应的同时运行,提高了能量守恒(〜10 kJ mol-1 H2)。我们的分析表明,铁氧还蛋白E'在很大程度上决定了分叉反应对pH2的敏感性,对还原/氧化电子载流子比例的调节对平衡移动的贡献较小。将氢化酶热力学数据与典型的和非典型的糖酵解途径整合在一起,以评估达到的“ Thauer极限”(每个葡萄糖4 H2)与温度和pH2的关系。例如,如果pH 2≤〜10 mbar,则分叉的[FeFe]-加氢酶反应允许在60°C下的Tauer极限。该结果还预测,表达非典型糖酵解途径的古生菌将不会从分叉的[FeFe]-氢化酶反应中受益。有趣的是,在实验中没有用[FeFe]氢化酶观察到古细菌。











































 京公网安备 11010802027423号
京公网安备 11010802027423号