当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rapid Biophysical Characterization and NMR Spectroscopy Structural Analysis of Small Proteins from Bacteria and Archaea.
ChemBioChem ( IF 2.6 ) Pub Date : 2020-01-21 , DOI: 10.1002/cbic.201900677 Nina Kubatova 1 , Dennis J Pyper 1 , Hendrik R A Jonker 1 , Krishna Saxena 1 , Laura Remmel 1 , Christian Richter 1 , Sabine Brantl 2 , Elena Evguenieva-Hackenberg 3 , Wolfgang R Hess 4 , Gabriele Klug 3 , Anita Marchfelder 5 , Jörg Soppa 6 , Wolfgang Streit 7 , Maxim Mayzel 8 , Vladislav Y Orekhov 8, 9 , Monika Fuxreiter 10 , Ruth A Schmitz 11 , Harald Schwalbe 1
ChemBioChem ( IF 2.6 ) Pub Date : 2020-01-21 , DOI: 10.1002/cbic.201900677 Nina Kubatova 1 , Dennis J Pyper 1 , Hendrik R A Jonker 1 , Krishna Saxena 1 , Laura Remmel 1 , Christian Richter 1 , Sabine Brantl 2 , Elena Evguenieva-Hackenberg 3 , Wolfgang R Hess 4 , Gabriele Klug 3 , Anita Marchfelder 5 , Jörg Soppa 6 , Wolfgang Streit 7 , Maxim Mayzel 8 , Vladislav Y Orekhov 8, 9 , Monika Fuxreiter 10 , Ruth A Schmitz 11 , Harald Schwalbe 1
Affiliation
|
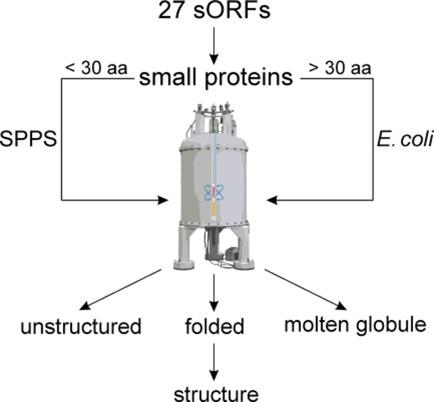
Proteins encoded by small open reading frames (sORFs) have a widespread occurrence in diverse microorganisms and can be of high functional importance. However, due to annotation biases and their technically challenging direct detection, these small proteins have been overlooked for a long time and were only recently rediscovered. The currently rapidly growing number of such proteins requires efficient methods to investigate their structure-function relationship. Herein, a method is presented for fast determination of the conformational properties of small proteins. Their small size makes them perfectly amenable for solution-state NMR spectroscopy. NMR spectroscopy can provide detailed information about their conformational states (folded, partially folded, and unstructured). In the context of the priority program on small proteins funded by the German research foundation (SPP2002), 27 small proteins from 9 different bacterial and archaeal organisms have been investigated. It is found that most of these small proteins are unstructured or partially folded. Bioinformatics tools predict that some of these unstructured proteins can potentially fold upon complex formation. A protocol for fast NMR spectroscopy structure elucidation is described for the small proteins that adopt a persistently folded structure by implementation of new NMR technologies, including automated resonance assignment and nonuniform sampling in combination with targeted acquisition.
中文翻译:

细菌和古细菌小蛋白的快速生物物理表征和核磁共振波谱结构分析。
由小型开放阅读框 (sORF) 编码的蛋白质广泛存在于多种微生物中,并且具有很高的功能重要性。然而,由于注释偏差及其直接检测在技术上具有挑战性,这些小蛋白质长期以来一直被忽视,直到最近才被重新发现。目前此类蛋白质的数量迅速增长,需要有效的方法来研究其结构与功能的关系。本文提出了一种快速测定小蛋白质构象特性的方法。它们的小尺寸使其非常适合溶液态核磁共振波谱。核磁共振波谱可以提供有关其构象状态(折叠、部分折叠和非结构化)的详细信息。在德国研究基金会 (SPP2002) 资助的小蛋白优先计划的背景下,对来自 9 种不同细菌和古细菌生物体的 27 种小蛋白进行了研究。研究发现,这些小蛋白质大多数是非结构化的或部分折叠的。生物信息学工具预测,其中一些非结构化蛋白质可能会在复杂形成时折叠。描述了通过实施新 NMR 技术(包括自动共振分配和与目标采集相结合的非均匀采样)而采用持续折叠结构的小蛋白质的快速 NMR 波谱结构阐明的协议。
更新日期:2020-01-21
中文翻译:

细菌和古细菌小蛋白的快速生物物理表征和核磁共振波谱结构分析。
由小型开放阅读框 (sORF) 编码的蛋白质广泛存在于多种微生物中,并且具有很高的功能重要性。然而,由于注释偏差及其直接检测在技术上具有挑战性,这些小蛋白质长期以来一直被忽视,直到最近才被重新发现。目前此类蛋白质的数量迅速增长,需要有效的方法来研究其结构与功能的关系。本文提出了一种快速测定小蛋白质构象特性的方法。它们的小尺寸使其非常适合溶液态核磁共振波谱。核磁共振波谱可以提供有关其构象状态(折叠、部分折叠和非结构化)的详细信息。在德国研究基金会 (SPP2002) 资助的小蛋白优先计划的背景下,对来自 9 种不同细菌和古细菌生物体的 27 种小蛋白进行了研究。研究发现,这些小蛋白质大多数是非结构化的或部分折叠的。生物信息学工具预测,其中一些非结构化蛋白质可能会在复杂形成时折叠。描述了通过实施新 NMR 技术(包括自动共振分配和与目标采集相结合的非均匀采样)而采用持续折叠结构的小蛋白质的快速 NMR 波谱结构阐明的协议。











































 京公网安备 11010802027423号
京公网安备 11010802027423号