当前位置:
X-MOL 学术
›
Spinal Cord
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Gene expression changes are associated with severe bone loss and deficient fracture callus formation in rats with complete spinal cord injury.
Spinal Cord ( IF 2.1 ) Pub Date : 2019-11-07 , DOI: 10.1038/s41393-019-0377-y Mariana M Butezloff 1 , José B Volpon 1 , João P B Ximenez 2 , Kelly Astolpho 1 , Vitor M Correlo 3 , Rui L Reis 3 , Raquel B Silva 4 , Ariane Zamarioli 1
Spinal Cord ( IF 2.1 ) Pub Date : 2019-11-07 , DOI: 10.1038/s41393-019-0377-y Mariana M Butezloff 1 , José B Volpon 1 , João P B Ximenez 2 , Kelly Astolpho 1 , Vitor M Correlo 3 , Rui L Reis 3 , Raquel B Silva 4 , Ariane Zamarioli 1
Affiliation
|
STUDY DESIGN
Animal study.
OBJECTIVES
To investigate the effects of SCI on bone quality and callus formation.
SETTING
University and hospital-based research center, Ribeirão Preto Medical School, Brazil.
METHODS
Rats sustaining a complete SCI for 10 days received a fracture at the femoral diaphysis and were followed-up for 14 days. Bone callus and contralateral nonfractured tibia were assessed by DXA, µCT, ELISA, histomorphometry, immunohistochemistry, biomechanical test, and gene expression.
RESULTS
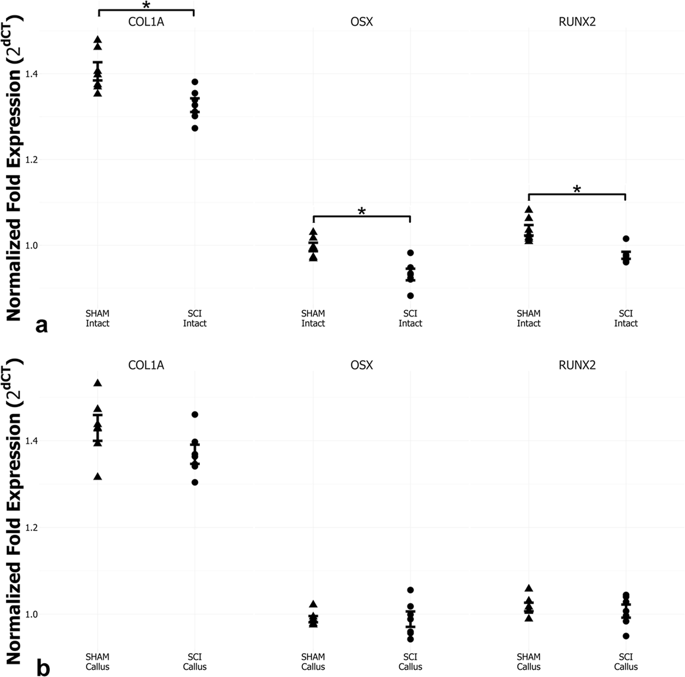
SCI downregulated osteoblastic-related gene expression in the nonfractured tibias, associated with a twofold increase in osteoclasts and overexpression of RANK/RANKL, which resulted in lower bone mass, impaired microarchitecture, and weaker bones. On day 14 postfracture, we revealed early and increased trabecular formation in the callus of SCI rats, despite a marked 75% decrease in OPG-positive cells, and 41% decrease in density. Furthermore, these calluses showed higher porosity and thinner newly formed trabeculae, leading to lower strength and angle failure.
CONCLUSIONS
SCI-induced bone loss resulted from increased bone resorption and decreased bone formation. We also evidenced accelerated bone healing in the SCI rats, which may be attributed to the predominant intramembranous ossification. However, the newly formed bone was thinner, less dense, and more porous than those in the non-SCI rats. As a result, these calluses are weaker and tolerate lesser torsion deformation than the controls, which may result in recurrent fractures and characterizes a remarkable feature that may severely impair life quality.
中文翻译:

基因表达的改变与完全性脊髓损伤大鼠的严重骨质流失和骨折call形成不足有关。
研究设计动物研究。目的探讨脊髓损伤对骨质和愈伤组织形成的影响。地点巴西里贝朗·普雷图医学院的大学和医院研究中心。方法维持10天完全SCI的大鼠在股骨干骨处骨折,并随访14天。通过DXA,µCT,ELISA,组织形态计量学,免疫组织化学,生物力学测试和基因表达来评估骨和对侧未骨折胫骨。结果SCI下调了未骨折胫骨中成骨细胞相关基因的表达,这与破骨细胞的增加和RANK / RANKL的过表达增加了两倍有关,这导致骨量降低,微结构受损和骨骼变弱。骨折后第14天,我们发现,尽管OPG阳性细胞显着降低了75%,密度降低了41%,但SCI大鼠愈伤组织中的小梁形成早期并增加了。此外,这些老茧显示出较高的孔隙率和较薄的新形成的小梁,从而导致较低的强度和角度破坏。结论SCI引起的骨质流失是由于骨吸收增加和骨形成减少所致。我们还证明了SCI大鼠骨骼加速愈合,这可能归因于膜内骨化。但是,新形成的骨骼比非SCI大鼠的骨骼更薄,密度更小且孔隙更多。结果,这些老茧比对照组更弱并且可以承受较小的扭转变形,这可能导致复发性骨折,并具有显着的特征,可能严重损害生活质量。尽管OPG阳性细胞显着降低了75%,密度降低了41%。此外,这些老茧显示出较高的孔隙率和较薄的新形成的小梁,从而导致较低的强度和角度破坏。结论SCI引起的骨丢失是由于骨吸收增加和骨形成减少所致。我们还证明了SCI大鼠骨骼加速愈合,这可能归因于膜内骨化。但是,新形成的骨骼比非SCI大鼠的骨骼更薄,密度更小且孔隙更多。结果,这些老茧比对照组更弱并且可以承受较小的扭转变形,这可能导致复发性骨折,并具有显着的特征,可能严重损害生活质量。尽管OPG阳性细胞显着降低了75%,密度降低了41%。此外,这些老茧显示出较高的孔隙率和较薄的新形成的小梁,从而导致较低的强度和角度破坏。结论SCI引起的骨丢失是由于骨吸收增加和骨形成减少所致。我们还证明了SCI大鼠骨骼加速愈合,这可能归因于膜内骨化。但是,新形成的骨骼比非SCI大鼠的骨骼更薄,密度更小且孔隙更多。结果,这些老茧比对照组更弱并且可以承受较小的扭转变形,这可能导致复发性骨折,并具有显着的特征,可能严重损害生活质量。此外,这些老茧显示出较高的孔隙率和较薄的新形成的小梁,从而导致较低的强度和角度破坏。结论SCI引起的骨质流失是由于骨吸收增加和骨形成减少所致。我们还证明了SCI大鼠骨骼加速愈合,这可能归因于膜内骨化。但是,新形成的骨骼比非SCI大鼠的骨骼更薄,密度更小且孔隙更多。结果,这些老茧比对照组更弱并且可以承受较小的扭转变形,这可能导致复发性骨折,并具有显着的特征,可能严重损害生活质量。此外,这些老茧显示出较高的孔隙率和较薄的新形成的小梁,从而导致较低的强度和角度破坏。结论SCI引起的骨质流失是由于骨吸收增加和骨形成减少所致。我们还证明了SCI大鼠骨骼加速愈合,这可能归因于膜内骨化。但是,新形成的骨骼比非SCI大鼠的骨骼更薄,密度更小且孔隙更多。结果,这些老茧比对照组更弱并且可以承受较小的扭转变形,这可能导致复发性骨折,并具有显着的特征,可能严重损害生活质量。结论SCI引起的骨质流失是由于骨吸收增加和骨形成减少所致。我们还证明了SCI大鼠骨骼加速愈合,这可能归因于膜内骨化。但是,新形成的骨骼比非SCI大鼠的骨骼更薄,密度更小且孔隙更多。结果,这些老茧比对照组更弱并且可以承受较小的扭转变形,这可能导致复发性骨折,并且具有显着的特征,可能严重损害生活质量。结论SCI引起的骨质流失是由于骨吸收增加和骨形成减少所致。我们还证明了SCI大鼠骨骼加速愈合,这可能归因于膜内骨化。但是,新形成的骨骼比非SCI大鼠的骨骼更薄,密度更小且孔隙更多。结果,这些老茧比对照组更弱并且可以承受较小的扭转变形,这可能导致复发性骨折,并具有显着的特征,可能严重损害生活质量。
更新日期:2019-11-08
中文翻译:

基因表达的改变与完全性脊髓损伤大鼠的严重骨质流失和骨折call形成不足有关。
研究设计动物研究。目的探讨脊髓损伤对骨质和愈伤组织形成的影响。地点巴西里贝朗·普雷图医学院的大学和医院研究中心。方法维持10天完全SCI的大鼠在股骨干骨处骨折,并随访14天。通过DXA,µCT,ELISA,组织形态计量学,免疫组织化学,生物力学测试和基因表达来评估骨和对侧未骨折胫骨。结果SCI下调了未骨折胫骨中成骨细胞相关基因的表达,这与破骨细胞的增加和RANK / RANKL的过表达增加了两倍有关,这导致骨量降低,微结构受损和骨骼变弱。骨折后第14天,我们发现,尽管OPG阳性细胞显着降低了75%,密度降低了41%,但SCI大鼠愈伤组织中的小梁形成早期并增加了。此外,这些老茧显示出较高的孔隙率和较薄的新形成的小梁,从而导致较低的强度和角度破坏。结论SCI引起的骨质流失是由于骨吸收增加和骨形成减少所致。我们还证明了SCI大鼠骨骼加速愈合,这可能归因于膜内骨化。但是,新形成的骨骼比非SCI大鼠的骨骼更薄,密度更小且孔隙更多。结果,这些老茧比对照组更弱并且可以承受较小的扭转变形,这可能导致复发性骨折,并具有显着的特征,可能严重损害生活质量。尽管OPG阳性细胞显着降低了75%,密度降低了41%。此外,这些老茧显示出较高的孔隙率和较薄的新形成的小梁,从而导致较低的强度和角度破坏。结论SCI引起的骨丢失是由于骨吸收增加和骨形成减少所致。我们还证明了SCI大鼠骨骼加速愈合,这可能归因于膜内骨化。但是,新形成的骨骼比非SCI大鼠的骨骼更薄,密度更小且孔隙更多。结果,这些老茧比对照组更弱并且可以承受较小的扭转变形,这可能导致复发性骨折,并具有显着的特征,可能严重损害生活质量。尽管OPG阳性细胞显着降低了75%,密度降低了41%。此外,这些老茧显示出较高的孔隙率和较薄的新形成的小梁,从而导致较低的强度和角度破坏。结论SCI引起的骨丢失是由于骨吸收增加和骨形成减少所致。我们还证明了SCI大鼠骨骼加速愈合,这可能归因于膜内骨化。但是,新形成的骨骼比非SCI大鼠的骨骼更薄,密度更小且孔隙更多。结果,这些老茧比对照组更弱并且可以承受较小的扭转变形,这可能导致复发性骨折,并具有显着的特征,可能严重损害生活质量。此外,这些老茧显示出较高的孔隙率和较薄的新形成的小梁,从而导致较低的强度和角度破坏。结论SCI引起的骨质流失是由于骨吸收增加和骨形成减少所致。我们还证明了SCI大鼠骨骼加速愈合,这可能归因于膜内骨化。但是,新形成的骨骼比非SCI大鼠的骨骼更薄,密度更小且孔隙更多。结果,这些老茧比对照组更弱并且可以承受较小的扭转变形,这可能导致复发性骨折,并具有显着的特征,可能严重损害生活质量。此外,这些老茧显示出较高的孔隙率和较薄的新形成的小梁,从而导致较低的强度和角度破坏。结论SCI引起的骨质流失是由于骨吸收增加和骨形成减少所致。我们还证明了SCI大鼠骨骼加速愈合,这可能归因于膜内骨化。但是,新形成的骨骼比非SCI大鼠的骨骼更薄,密度更小且孔隙更多。结果,这些老茧比对照组更弱并且可以承受较小的扭转变形,这可能导致复发性骨折,并具有显着的特征,可能严重损害生活质量。结论SCI引起的骨质流失是由于骨吸收增加和骨形成减少所致。我们还证明了SCI大鼠骨骼加速愈合,这可能归因于膜内骨化。但是,新形成的骨骼比非SCI大鼠的骨骼更薄,密度更小且孔隙更多。结果,这些老茧比对照组更弱并且可以承受较小的扭转变形,这可能导致复发性骨折,并且具有显着的特征,可能严重损害生活质量。结论SCI引起的骨质流失是由于骨吸收增加和骨形成减少所致。我们还证明了SCI大鼠骨骼加速愈合,这可能归因于膜内骨化。但是,新形成的骨骼比非SCI大鼠的骨骼更薄,密度更小且孔隙更多。结果,这些老茧比对照组更弱并且可以承受较小的扭转变形,这可能导致复发性骨折,并具有显着的特征,可能严重损害生活质量。











































 京公网安备 11010802027423号
京公网安备 11010802027423号