当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploring the Molecular Mechanisms Underlying the in vitro Anticancer Effects of Multitarget-Directed Hydrazone Ruthenium(II)-Arene Complexes.
ChemMedChem ( IF 3.6 ) Pub Date : 2019-11-08 , DOI: 10.1002/cmdc.201900551 Massimiliano Cuccioloni 1 , Laura Bonfili 1 , Valentina Cecarini 1 , Massimo Nabissi 2 , Riccardo Pettinari 2 , Fabio Marchetti 3 , Riccardo Petrelli 2 , Loredana Cappellacci 2 , Mauro Angeletti 1 , Anna Maria Eleuteri 1
ChemMedChem ( IF 3.6 ) Pub Date : 2019-11-08 , DOI: 10.1002/cmdc.201900551 Massimiliano Cuccioloni 1 , Laura Bonfili 1 , Valentina Cecarini 1 , Massimo Nabissi 2 , Riccardo Pettinari 2 , Fabio Marchetti 3 , Riccardo Petrelli 2 , Loredana Cappellacci 2 , Mauro Angeletti 1 , Anna Maria Eleuteri 1
Affiliation
|
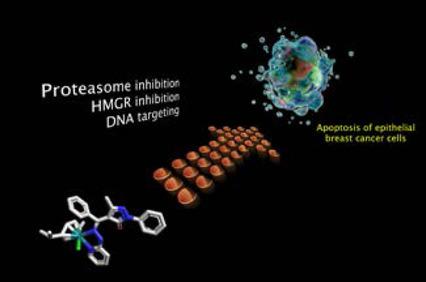
The molecular targets and the modes of action behind the cytotoxicity of two structurally established N,O- or N,N-hydrazone ruthenium(II)-arene complexes were explored in human breast adenocarcinoma cells (MCF-7) and paralleled in non-cancerous and cisplatin-resistant counterparts (MCF-10A and MCF-7CR respectively). Both complexes, [Ru(hmb)(L1)Cl] (1, L1=4-((2-(2,4-dinitrophenyl)hydrazono)(phenyl)methyl)-3-methyl-1-phenyl-1H-pyrazol-5-olate) and [Ru(cym)(L2)Cl] (2, L2=1-((3-methyl-5-oxo-1-phenyl-1H-pyrazol-4(5H)-ylidene)(phenyl)methyl)-2-(pyridin-2-yl)hydrazin-1-ide), reversibly interact with moderate-to-high affinity with a number of molecular targets in cell-free assays, namely serum albumin, DNA, the 20S proteasome and hydroxymethylglutaryl-CoA reductase. Most interestingly, only 2 readily crosses the cell membrane and preserves its binding/modulatory ability toward the targets of interest upon rapid cellular internalization. The resulting action at multiple levels of the cancer cascade is likely the cause for the selective sensitization of tumour cells to p27-mediated apoptotic death, and for the ability of 2 to overcome the drug resistance problem.
中文翻译:

探索分子靶向机制的多靶点定向Hy Hy(Ar)络合物的体外抗癌作用。
在人乳腺癌细胞(MCF-7)中探索了两种结构确定的N,O-或N,N-hydr钌(II)-芳烃配合物的分子靶标和细胞毒性背后的作用模式,并在非癌性中进行了平行研究和顺铂耐药的对应物(分别为MCF-10A和MCF-7CR)。两种配合物[Ru(hmb)(L1)Cl](1,L1 = 4-((2-(2,4-二硝基苯基)肼基)(苯基)甲基)-3-甲基-1-苯基-1H-吡唑-5-olate)和[Ru(cym)(L2)Cl](2,L2 = 1-((3-甲基-5-氧代-1-苯基-1H-吡唑-4(5H)-亚烷基)(苯基)甲基)-2-(吡啶-2-基)肼-1-基)在无细胞测定中与多种分子靶标(包括血清白蛋白,DNA,20S蛋白酶体)可逆地相互作用,具有中至高亲和力和羟甲基戊二酰辅酶A还原酶。最有趣的是 在细胞快速内化后,仅有2个分子容易穿过细胞膜并保持其对目标靶标的结合/调节能力。在多种癌症级联反应中产生的作用可能是肿瘤细胞对p27介导的细胞凋亡死亡选择性敏化的原因,也是2克服药物耐药性问题的能力的原因。
更新日期:2019-11-18
中文翻译:

探索分子靶向机制的多靶点定向Hy Hy(Ar)络合物的体外抗癌作用。
在人乳腺癌细胞(MCF-7)中探索了两种结构确定的N,O-或N,N-hydr钌(II)-芳烃配合物的分子靶标和细胞毒性背后的作用模式,并在非癌性中进行了平行研究和顺铂耐药的对应物(分别为MCF-10A和MCF-7CR)。两种配合物[Ru(hmb)(L1)Cl](1,L1 = 4-((2-(2,4-二硝基苯基)肼基)(苯基)甲基)-3-甲基-1-苯基-1H-吡唑-5-olate)和[Ru(cym)(L2)Cl](2,L2 = 1-((3-甲基-5-氧代-1-苯基-1H-吡唑-4(5H)-亚烷基)(苯基)甲基)-2-(吡啶-2-基)肼-1-基)在无细胞测定中与多种分子靶标(包括血清白蛋白,DNA,20S蛋白酶体)可逆地相互作用,具有中至高亲和力和羟甲基戊二酰辅酶A还原酶。最有趣的是 在细胞快速内化后,仅有2个分子容易穿过细胞膜并保持其对目标靶标的结合/调节能力。在多种癌症级联反应中产生的作用可能是肿瘤细胞对p27介导的细胞凋亡死亡选择性敏化的原因,也是2克服药物耐药性问题的能力的原因。











































 京公网安备 11010802027423号
京公网安备 11010802027423号