Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CNS myelin protein 36K regulates oligodendrocyte differentiation through Notch.
Glia ( IF 5.4 ) Pub Date : 2019-11-08 , DOI: 10.1002/glia.23732 Bhuvaneswari Nagarajan 1 , Alexander Harder 2 , Anna Japp 3 , Felix Häberlein 1, 4 , Enrico Mingardo 1 , Henning Kleinert 1 , Öznur Yilmaz 1 , Angelika Zoons 5 , Birgit Rau 5 , Andrea Christ 1 , Ulrich Kubitscheck 2 , Britta Eiberger 1 , Roger Sandhoff 6 , Matthias Eckhardt 7 , Dieter Hartmann 5 , Benjamin Odermatt 1, 5
Glia ( IF 5.4 ) Pub Date : 2019-11-08 , DOI: 10.1002/glia.23732 Bhuvaneswari Nagarajan 1 , Alexander Harder 2 , Anna Japp 3 , Felix Häberlein 1, 4 , Enrico Mingardo 1 , Henning Kleinert 1 , Öznur Yilmaz 1 , Angelika Zoons 5 , Birgit Rau 5 , Andrea Christ 1 , Ulrich Kubitscheck 2 , Britta Eiberger 1 , Roger Sandhoff 6 , Matthias Eckhardt 7 , Dieter Hartmann 5 , Benjamin Odermatt 1, 5
Affiliation
|
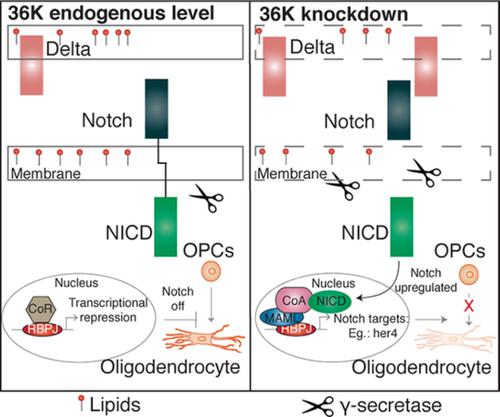
In contrast to humans and other mammals, zebrafish can successfully regenerate and remyelinate central nervous system (CNS) axons following injury. In addition to common myelin proteins found in mammalian myelin, 36K protein is a major component of teleost fish CNS myelin. Although 36K is one of the most abundant proteins in zebrafish brain, its function remains unknown. Here we investigate the function of 36K using translation-blocking Morpholinos. Morphant larvae showed fewer dorsally migrated oligodendrocyte precursor cells as well as upregulation of Notch ligand. A gamma secretase inhibitor, which prevents activation of Notch, could rescue oligodendrocyte precursor cell numbers in 36K morphants, suggesting that 36K regulates initial myelination through inhibition of Notch signaling. Since 36K like other short chain dehydrogenases might act on lipids, we performed thin layer chromatography and mass spectrometry of lipids and found changes in lipid composition in 36K morphant larvae. Altogether, we suggest that during early development 36K regulates membrane lipid composition, thereby altering the amount of transmembrane Notch ligands and the efficiency of intramembrane gamma secretase processing of Notch and thereby influencing oligodendrocyte precursor cell differentiation and further myelination. Further studies on the role of 36K short chain dehydrogenase in oligodendrocyte precursor cell differentiation during remyelination might open up new strategies for remyelination therapies in human patients.
中文翻译:

CNS髓磷脂蛋白36K通过Notch调节少突胶质细胞的分化。
与人类和其他哺乳动物相反,斑马鱼在受伤后可以成功地再生和重新使中枢神经系统(CNS)轴突再生。除哺乳动物髓磷脂中发现的常见髓磷脂蛋白外,36K蛋白是硬骨鱼中枢神经系统髓磷脂的主要成分。尽管36K是斑马鱼大脑中最丰富的蛋白质之一,但其功能仍然未知。在这里,我们使用阻断翻译的Morpholinos来研究36K的功能。Morphant幼虫显示较少的背向迁移的少突胶质细胞前体细胞以及Notch配体的上调。可以阻止Notch活化的伽马分泌酶抑制剂可以挽救36K morphant中少突胶质前体细胞的数量,这表明36K通过抑制Notch信号传导来调节初始髓鞘形成。由于36K像其他短链脱氢酶一样可能作用于脂质,因此我们对脂质进行了薄层色谱分析和质谱分析,发现36K morphant幼虫的脂质组成发生了变化。总而言之,我们建议在早期发育过程中36K调节膜脂质的组成,从而改变跨膜Notch配体的数量和Notch膜内γ分泌酶加工的效率,从而影响少突胶质前体细胞的分化和进一步的髓鞘形成。关于36K短链脱氢酶在髓鞘再生过程中少突胶质前体细胞分化中的作用的进一步研究可能会为人类患者的髓鞘再生治疗开辟新的策略。我们进行了脂质的薄层色谱分析和质谱分析,发现36K morphant幼虫的脂质组成发生了变化。总而言之,我们建议在早期发育过程中36K调节膜脂质的组成,从而改变跨膜Notch配体的数量和Notch膜内γ分泌酶加工的效率,从而影响少突胶质前体细胞的分化和进一步的髓鞘形成。关于36K短链脱氢酶在髓鞘再生过程中少突胶质前体细胞分化中的作用的进一步研究可能会为人类患者的髓鞘再生治疗开辟新的策略。我们进行了脂质的薄层色谱分析和质谱分析,发现36K morphant幼虫的脂质组成发生了变化。总而言之,我们建议在早期发育过程中36K调节膜脂质的组成,从而改变跨膜Notch配体的数量和Notch膜内γ分泌酶加工的效率,从而影响少突胶质前体细胞的分化和进一步的髓鞘形成。关于36K短链脱氢酶在髓鞘再生过程中少突胶质前体细胞分化中的作用的进一步研究可能会为人类患者的髓鞘再生治疗开辟新的策略。从而改变跨膜Notch配体的量和Notch的膜内γ分泌酶加工的效率,从而影响少突胶质前体细胞的分化和进一步的髓鞘形成。关于36K短链脱氢酶在髓鞘再生过程中少突胶质前体细胞分化中的作用的进一步研究可能会为人类患者的髓鞘再生治疗开辟新的策略。从而改变跨膜Notch配体的量和Notch的膜内γ分泌酶加工的效率,从而影响少突胶质前体细胞的分化和进一步的髓鞘形成。关于36K短链脱氢酶在髓鞘再生过程中少突胶质前体细胞分化中的作用的进一步研究可能会为人类患者的髓鞘再生治疗开辟新的策略。
更新日期:2019-11-08
中文翻译:

CNS髓磷脂蛋白36K通过Notch调节少突胶质细胞的分化。
与人类和其他哺乳动物相反,斑马鱼在受伤后可以成功地再生和重新使中枢神经系统(CNS)轴突再生。除哺乳动物髓磷脂中发现的常见髓磷脂蛋白外,36K蛋白是硬骨鱼中枢神经系统髓磷脂的主要成分。尽管36K是斑马鱼大脑中最丰富的蛋白质之一,但其功能仍然未知。在这里,我们使用阻断翻译的Morpholinos来研究36K的功能。Morphant幼虫显示较少的背向迁移的少突胶质细胞前体细胞以及Notch配体的上调。可以阻止Notch活化的伽马分泌酶抑制剂可以挽救36K morphant中少突胶质前体细胞的数量,这表明36K通过抑制Notch信号传导来调节初始髓鞘形成。由于36K像其他短链脱氢酶一样可能作用于脂质,因此我们对脂质进行了薄层色谱分析和质谱分析,发现36K morphant幼虫的脂质组成发生了变化。总而言之,我们建议在早期发育过程中36K调节膜脂质的组成,从而改变跨膜Notch配体的数量和Notch膜内γ分泌酶加工的效率,从而影响少突胶质前体细胞的分化和进一步的髓鞘形成。关于36K短链脱氢酶在髓鞘再生过程中少突胶质前体细胞分化中的作用的进一步研究可能会为人类患者的髓鞘再生治疗开辟新的策略。我们进行了脂质的薄层色谱分析和质谱分析,发现36K morphant幼虫的脂质组成发生了变化。总而言之,我们建议在早期发育过程中36K调节膜脂质的组成,从而改变跨膜Notch配体的数量和Notch膜内γ分泌酶加工的效率,从而影响少突胶质前体细胞的分化和进一步的髓鞘形成。关于36K短链脱氢酶在髓鞘再生过程中少突胶质前体细胞分化中的作用的进一步研究可能会为人类患者的髓鞘再生治疗开辟新的策略。我们进行了脂质的薄层色谱分析和质谱分析,发现36K morphant幼虫的脂质组成发生了变化。总而言之,我们建议在早期发育过程中36K调节膜脂质的组成,从而改变跨膜Notch配体的数量和Notch膜内γ分泌酶加工的效率,从而影响少突胶质前体细胞的分化和进一步的髓鞘形成。关于36K短链脱氢酶在髓鞘再生过程中少突胶质前体细胞分化中的作用的进一步研究可能会为人类患者的髓鞘再生治疗开辟新的策略。从而改变跨膜Notch配体的量和Notch的膜内γ分泌酶加工的效率,从而影响少突胶质前体细胞的分化和进一步的髓鞘形成。关于36K短链脱氢酶在髓鞘再生过程中少突胶质前体细胞分化中的作用的进一步研究可能会为人类患者的髓鞘再生治疗开辟新的策略。从而改变跨膜Notch配体的量和Notch的膜内γ分泌酶加工的效率,从而影响少突胶质前体细胞的分化和进一步的髓鞘形成。关于36K短链脱氢酶在髓鞘再生过程中少突胶质前体细胞分化中的作用的进一步研究可能会为人类患者的髓鞘再生治疗开辟新的策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号