当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of new lophine-carbohydrate hybrids as cholinesterase inhibitors: cytotoxicity evaluation and molecular modeling.
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2019-11-08 , DOI: 10.1039/c9md00358d João Paulo Bizarro Lopes 1 , Luana Silva 1 , Marco Antonio Ceschi 1 , Diogo Seibert Lüdtke 1 , Aline Rigon Zimmer 2 , Thais Carine Ruaro 2 , Rafael Ferreira Dantas 3 , Cristiane Martins Cardoso de Salles 4 , Floriano Paes Silva-Jr 3 , Mario Roberto Senger 3 , Gisele Barbosa 5 , Lídia Moreira Lima 5 , Isabella Alvim Guedes 6 , Laurent Emmanuel Dardenne 6
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2019-11-08 , DOI: 10.1039/c9md00358d João Paulo Bizarro Lopes 1 , Luana Silva 1 , Marco Antonio Ceschi 1 , Diogo Seibert Lüdtke 1 , Aline Rigon Zimmer 2 , Thais Carine Ruaro 2 , Rafael Ferreira Dantas 3 , Cristiane Martins Cardoso de Salles 4 , Floriano Paes Silva-Jr 3 , Mario Roberto Senger 3 , Gisele Barbosa 5 , Lídia Moreira Lima 5 , Isabella Alvim Guedes 6 , Laurent Emmanuel Dardenne 6
Affiliation
|
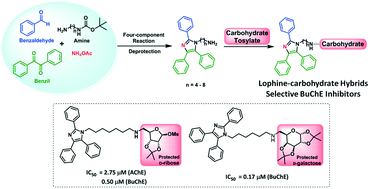
In this study, we synthesized nine novel hybrids derived from D-xylose, D-ribose, and D-galactose sugars connected by a methylene chain with lophine. The compounds were synthesized by a four-component reaction to afford the substituted imidazole moiety, followed by the displacement reaction between sugar derivatives with an appropriate N-alkylamino-lophine. All the compounds were found to be the potent and selective inhibitors of BuChE activity in mouse serum, with compound 9a (a D-galactose derivative) being the most potent inhibitor (IC50 = 0.17 μM). According to the molecular modeling results, all the compounds indicated that the lophine moiety existed at the bottom of the BuChE cavity and formed a T-stacking interaction with Trp231, a residue accessible exclusively in the BuChE cavity. Noteworthily, only one compound exhibited activity against AChE (8b; IC50 = 2.75 μM). Moreover, the in silico ADME predictions indicated that all the hybrids formulated in this study were drug-likely, orally available, and able to reach the CNS. Further, in vitro studies demonstrated that the two most potent compounds against BuChE (8b and 9a) had no cytotoxic effects in the Vero (kidney), HepG2 (hepatic), and C6 (astroglial) cell lines.
中文翻译:

作为胆碱酯酶抑制剂的新型洛芬碱-碳水化合物杂化物的合成:细胞毒性评估和分子建模。
在这项研究中,我们合成了九种新型杂合体,这些杂合体源自通过亚甲基链与洛芬碱连接的D-木糖、 D-核糖和D-半乳糖。该化合物通过四组分反应合成,得到取代的咪唑部分,然后进行糖衍生物与适当的N-烷基氨基-洛芬之间的置换反应。所有化合物均被发现是小鼠血清中 BuChE 活性的有效和选择性抑制剂,其中化合物9a ( D-半乳糖衍生物)是最有效的抑制剂(IC 50 = 0.17 μM)。根据分子模拟结果,所有化合物均表明 Lophine 部分存在于 BuChE 空腔底部,并与 Trp231(一种仅在 BuChE 空腔中可接近的残基)形成 T 堆积相互作用。值得注意的是,只有一种化合物表现出抗 AChE 活性( 8b ;IC 50 = 2.75 μM)。此外,计算机ADME 预测表明,本研究中配制的所有混合药物均具有药物作用、可口服且能够到达中枢神经系统。此外,体外研究表明,两种最有效的 BuChE 化合物( 8b和9a )在 Vero(肾)、HepG2(肝)和 C6(星形胶质细胞)细胞系中没有细胞毒性作用。
更新日期:2019-12-13
中文翻译:

作为胆碱酯酶抑制剂的新型洛芬碱-碳水化合物杂化物的合成:细胞毒性评估和分子建模。
在这项研究中,我们合成了九种新型杂合体,这些杂合体源自通过亚甲基链与洛芬碱连接的D-木糖、 D-核糖和D-半乳糖。该化合物通过四组分反应合成,得到取代的咪唑部分,然后进行糖衍生物与适当的N-烷基氨基-洛芬之间的置换反应。所有化合物均被发现是小鼠血清中 BuChE 活性的有效和选择性抑制剂,其中化合物9a ( D-半乳糖衍生物)是最有效的抑制剂(IC 50 = 0.17 μM)。根据分子模拟结果,所有化合物均表明 Lophine 部分存在于 BuChE 空腔底部,并与 Trp231(一种仅在 BuChE 空腔中可接近的残基)形成 T 堆积相互作用。值得注意的是,只有一种化合物表现出抗 AChE 活性( 8b ;IC 50 = 2.75 μM)。此外,计算机ADME 预测表明,本研究中配制的所有混合药物均具有药物作用、可口服且能够到达中枢神经系统。此外,体外研究表明,两种最有效的 BuChE 化合物( 8b和9a )在 Vero(肾)、HepG2(肝)和 C6(星形胶质细胞)细胞系中没有细胞毒性作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号