当前位置:
X-MOL 学术
›
Lancet Neurol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A support programme for secondary prevention in patients with transient ischaemic attack and minor stroke (INSPiRE-TMS): an open-label, randomised controlled trial
The Lancet Neurology ( IF 46.5 ) Pub Date : 2020-01-01 , DOI: 10.1016/s1474-4422(19)30369-2 Michael Ahmadi 1 , Inga Laumeier 2 , Thomas Ihl 2 , Maureen Steinicke 2 , Caroline Ferse 2 , Matthias Endres 3 , Armin Grau 4 , Sidsel Hastrup 5 , Holger Poppert 6 , Frederick Palm 4 , Martin Schoene 2 , Christian L Seifert 6 , Farid I Kandil 7 , Joachim E Weber 1 , Paul von Weitzel-Mudersbach 5 , Martin L J Wimmer 8 , Ale Algra 9 , Pierre Amarenco 10 , Jacoba P Greving 9 , Otto Busse 11 , Friedrich Köhler 12 , Peter Marx 2 , Heinrich J Audebert 13
The Lancet Neurology ( IF 46.5 ) Pub Date : 2020-01-01 , DOI: 10.1016/s1474-4422(19)30369-2 Michael Ahmadi 1 , Inga Laumeier 2 , Thomas Ihl 2 , Maureen Steinicke 2 , Caroline Ferse 2 , Matthias Endres 3 , Armin Grau 4 , Sidsel Hastrup 5 , Holger Poppert 6 , Frederick Palm 4 , Martin Schoene 2 , Christian L Seifert 6 , Farid I Kandil 7 , Joachim E Weber 1 , Paul von Weitzel-Mudersbach 5 , Martin L J Wimmer 8 , Ale Algra 9 , Pierre Amarenco 10 , Jacoba P Greving 9 , Otto Busse 11 , Friedrich Köhler 12 , Peter Marx 2 , Heinrich J Audebert 13
Affiliation
|
BACKGROUND
Patients with recent stroke or transient ischaemic attack are at high risk for a further vascular event, possibly leading to permanent disability or death. Although evidence-based treatments for secondary prevention are available, many patients do not achieve recommended behavioural modifications and pharmaceutical prevention targets in the long-term. We aimed to investigate whether a support programme for enhanced secondary prevention can reduce the frequency of recurrent vascular events. METHODS
INSPiRE-TMS was an open-label, multicentre, international randomised controlled trial done at seven German hospitals with acute stroke units and a Danish stroke centre. Patients with non-disabling stroke or transient ischaemic attack within 2 weeks from study enrolment and at least one modifiable risk factor (ie, arterial hypertension, diabetes, atrial fibrillation, or smoking) were included. Computerised randomisation was used to allocate patients (1:1) either to the support programme in addition to conventional care or to conventional care alone. The support programme used feedback and motivational interviewing strategies with eight outpatient visits over 2 years aiming to improve adherence to secondary prevention targets. The primary outcome was the composite of major vascular events consisting of stroke, acute coronary syndrome, and vascular death, assessed in the intention-to-treat population (all patients who underwent randomisation, did not withdraw study participation, and had at least one follow-up). Outcomes were assessed at annual follow-ups using time-to-first-event analysis. All-cause death was monitored as a safety outcome. This trial is registered with ClinicalTrials.gov, NCT01586702. FINDINGS
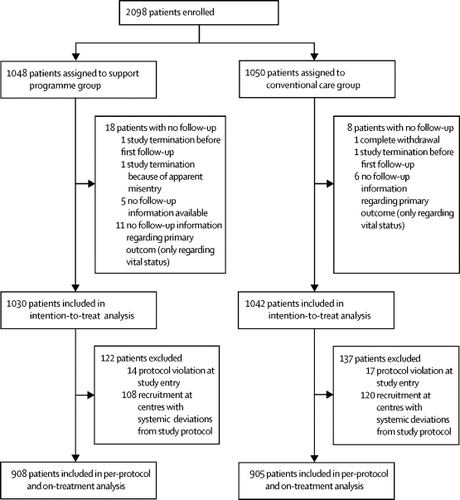
From Aug 22, 2011, to Oct 30, 2017, we enrolled 2098 patients. Of those, 1048 (50·0%) were randomly assigned to the support programme group and 1050 (50·0%) patients were assigned to the conventional care group. 1030 (98·3%) patients in the support group and 1042 (99·2%) patients in the conventional care group were included in the intention-to-treat analysis. The mean age of analysed participants was 67·4 years and 700 (34%) were women. After a mean follow-up of 3·6 years, the primary outcome of major vascular events had occurred in 163 (15·8%) of 1030 patients of the support programme group and in 175 (16·8%) of 1042 patients of the conventional care group (hazard ratio [HR] 0·92, 95% CI 0·75-1·14). Total major vascular event numbers were 209 for the support programme group and 225 for the conventional care group (incidence rate ratio 0·93, 95% CI 0·77-1·12; p=0·46) and all-cause death occurred in 73 (7·1%) patients in the support programme group and 85 (8·2%) patients in the conventional care group (HR 0·85, 0·62-1·17). More patients in the support programme group achieved secondary prevention targets (eg, in 1-year-follow-up 52% vs 42% [p<0·0001] for blood pressure, 62% vs 54% [p=0·0010] for LDL, 33% vs 19% [p<0·0001] for physical activity, and 51% vs 34% [p=0·0010] for smoking cessation). INTERPRETATION
Provision of an intensified secondary prevention programme in patients with non-disabling stroke or transient ischaemic attack was associated with improved achievement of secondary prevention targets but did not lead to a significantly lower rate of major vascular events. Further research is needed to investigate the effects of support programmes in selected patients who do not achieve secondary prevention targets soon after discharge. FUNDING
German Federal Ministry of Education and Research, Pfizer, and German Stroke Foundation.
中文翻译:

短暂性脑缺血发作和轻微卒中患者二级预防支持计划 (INSPiRE-TMS):一项开放标签、随机对照试验
背景近期中风或短暂性脑缺血发作的患者处于进一步血管事件的高风险中,可能导致永久性残疾或死亡。尽管可以进行二级预防的循证治疗,但从长远来看,许多患者并未达到推荐的行为改变和药物预防目标。我们旨在调查加强二级预防的支持计划是否可以减少血管事件复发的频率。方法 INSPiRE-TMS 是一项开放标签、多中心、国际随机对照试验,在七家设有急性卒中单元的德国医院和一家丹麦卒中中心进行。研究入选后 2 周内患有非致残性卒中或短暂性脑缺血发作且至少有一种可改变的危险因素(即动脉高血压、包括糖尿病、心房颤动或吸烟)。计算机随机化用于将患者 (1:1) 分配到常规护理之外的支持计划或单独的常规护理。该支持计划使用反馈和动机访谈策略,在 2 年内进行了 8 次门诊访问,旨在提高对二级预防目标的依从性。主要结局是在意向治疗人群中评估的主要血管事件,包括卒中、急性冠脉综合征和血管性死亡(所有接受随机分组、未退出研究且至少有一次随访的患者) -向上)。使用首次事件时间分析在年度随访中评估结果。全因死亡作为安全性结果进行监测。该试验已在 ClinicalTrials.gov 注册,NCT01586702。结果 从2011年8月22日至2017年10月30日,我们招募了2098名患者。其中,1048 (50·0%) 名患者被随机分配到支持计划组,1050 (50·0%) 名患者被分配到常规护理组。意向治疗分析包括支持组中的 1030 名 (98·3%) 患者和常规护理组中的 1042 名 (99·2%) 患者。被分析参与者的平均年龄为 67·4 岁,其中 700 人 (34%) 是女性。平均随访 3·6 年后,支持计划组 1030 名患者中的 163 名 (15·8%) 和 1042 名患者中的 175 名 (16·8%) 发生了主要血管事件的主要结果。常规护理组(风险比 [HR] 0·92,95% CI 0·75-1·14)。支持计划组的主要血管事件总数为 209 例,常规治疗组为 225 例(发生率比 0·93,95% CI 0·77-1·12;p=0·46)并且发生全因死亡在支持计划组的 73 (7·1%) 名患者和常规护理组的 85 (8·2%) 名患者中 (HR 0·85, 0·62-1·17)。支持计划组中更多患者达到二级预防目标(例如,在 1 年随访中,血压分别为 52% 与 42% [p<0·0001],62% 与 54% [p=0·0010]对于 LDL,33% 对 19% [p<0·0001] 用于体力活动,51% 对 34% [p=0·0010] 用于戒烟)。解释 为非致残性卒中或短暂性脑缺血发作患者提供强化二级预防计划与二级预防目标的实现提高相关,但并未显着降低主要血管事件的发生率。需要进一步的研究来调查支持计划对出院后没有立即达到二级预防目标的特定患者的影响。资助德国联邦教育和研究部、辉瑞和德国中风基金会。
更新日期:2020-01-01
中文翻译:

短暂性脑缺血发作和轻微卒中患者二级预防支持计划 (INSPiRE-TMS):一项开放标签、随机对照试验
背景近期中风或短暂性脑缺血发作的患者处于进一步血管事件的高风险中,可能导致永久性残疾或死亡。尽管可以进行二级预防的循证治疗,但从长远来看,许多患者并未达到推荐的行为改变和药物预防目标。我们旨在调查加强二级预防的支持计划是否可以减少血管事件复发的频率。方法 INSPiRE-TMS 是一项开放标签、多中心、国际随机对照试验,在七家设有急性卒中单元的德国医院和一家丹麦卒中中心进行。研究入选后 2 周内患有非致残性卒中或短暂性脑缺血发作且至少有一种可改变的危险因素(即动脉高血压、包括糖尿病、心房颤动或吸烟)。计算机随机化用于将患者 (1:1) 分配到常规护理之外的支持计划或单独的常规护理。该支持计划使用反馈和动机访谈策略,在 2 年内进行了 8 次门诊访问,旨在提高对二级预防目标的依从性。主要结局是在意向治疗人群中评估的主要血管事件,包括卒中、急性冠脉综合征和血管性死亡(所有接受随机分组、未退出研究且至少有一次随访的患者) -向上)。使用首次事件时间分析在年度随访中评估结果。全因死亡作为安全性结果进行监测。该试验已在 ClinicalTrials.gov 注册,NCT01586702。结果 从2011年8月22日至2017年10月30日,我们招募了2098名患者。其中,1048 (50·0%) 名患者被随机分配到支持计划组,1050 (50·0%) 名患者被分配到常规护理组。意向治疗分析包括支持组中的 1030 名 (98·3%) 患者和常规护理组中的 1042 名 (99·2%) 患者。被分析参与者的平均年龄为 67·4 岁,其中 700 人 (34%) 是女性。平均随访 3·6 年后,支持计划组 1030 名患者中的 163 名 (15·8%) 和 1042 名患者中的 175 名 (16·8%) 发生了主要血管事件的主要结果。常规护理组(风险比 [HR] 0·92,95% CI 0·75-1·14)。支持计划组的主要血管事件总数为 209 例,常规治疗组为 225 例(发生率比 0·93,95% CI 0·77-1·12;p=0·46)并且发生全因死亡在支持计划组的 73 (7·1%) 名患者和常规护理组的 85 (8·2%) 名患者中 (HR 0·85, 0·62-1·17)。支持计划组中更多患者达到二级预防目标(例如,在 1 年随访中,血压分别为 52% 与 42% [p<0·0001],62% 与 54% [p=0·0010]对于 LDL,33% 对 19% [p<0·0001] 用于体力活动,51% 对 34% [p=0·0010] 用于戒烟)。解释 为非致残性卒中或短暂性脑缺血发作患者提供强化二级预防计划与二级预防目标的实现提高相关,但并未显着降低主要血管事件的发生率。需要进一步的研究来调查支持计划对出院后没有立即达到二级预防目标的特定患者的影响。资助德国联邦教育和研究部、辉瑞和德国中风基金会。











































 京公网安备 11010802027423号
京公网安备 11010802027423号