当前位置:
X-MOL 学术
›
Bone Marrow Transpl.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ibrutinib as a salvage therapy after allogeneic HCT for chronic lymphocytic leukemia.
Bone Marrow Transplantation ( IF 4.5 ) Pub Date : 2019-11-07 , DOI: 10.1038/s41409-019-0742-7 Mauricette Michallet 1 , Peter Dreger 2 , Mohamad Sobh 1 , Linda Koster 3 , Jennifer Hoek 3 , Ariane Boumendil 4 , Christof Scheid 5 , Christopher P Fox 6 , Gerald Wulf 7 , William Krüger 8 , Michel van Gelder 9 , Paolo Corradini 10 , Domenico Russo 11 , Jakob Passweg 12 , Hélène Schoemans 13 , Wolfgang Bethge 14 , Nicolaas Schaap 15 , Jan Cornelissen 16 , Paul Browne 17 , Nadira Durakovic 18 , Lutz Muller 19 , Silvia Montoto 20 , Nicolaus Kroger 21 , Johannes Schetelig 22 ,
Bone Marrow Transplantation ( IF 4.5 ) Pub Date : 2019-11-07 , DOI: 10.1038/s41409-019-0742-7 Mauricette Michallet 1 , Peter Dreger 2 , Mohamad Sobh 1 , Linda Koster 3 , Jennifer Hoek 3 , Ariane Boumendil 4 , Christof Scheid 5 , Christopher P Fox 6 , Gerald Wulf 7 , William Krüger 8 , Michel van Gelder 9 , Paolo Corradini 10 , Domenico Russo 11 , Jakob Passweg 12 , Hélène Schoemans 13 , Wolfgang Bethge 14 , Nicolaas Schaap 15 , Jan Cornelissen 16 , Paul Browne 17 , Nadira Durakovic 18 , Lutz Muller 19 , Silvia Montoto 20 , Nicolaus Kroger 21 , Johannes Schetelig 22 ,
Affiliation
|
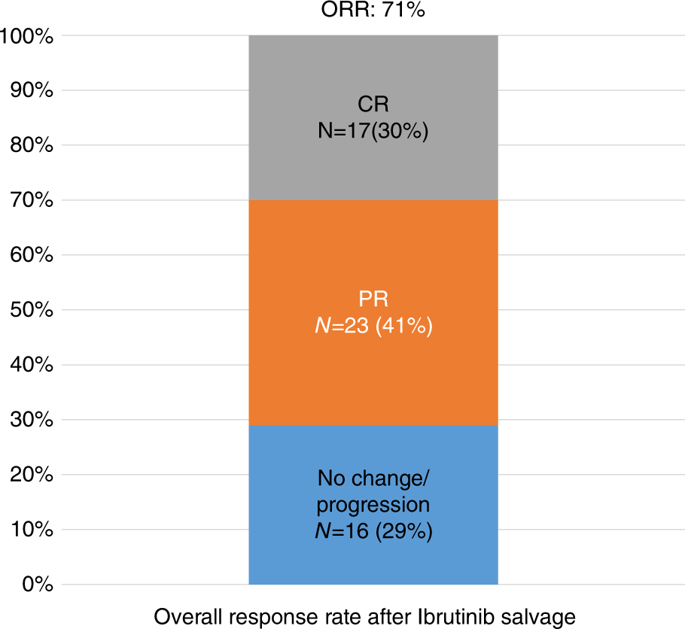
The purpose of our study is to provide information on safety and efficacy of ibrutinib as salvage treatment after allo-HSCT for CLL. A total of 56 patients were included, 36 (64%) males; median age at transplantation was 48 years (range: 35-64) and the median number of treatment lines prior to transplantation was 3 (1-10). The median time between allo-HSCT and Ibrutinib was 30 months (range: 1-140). Overall, 40 (71%) patients responded to Ibrutinib; 23 (41%) PR, and 17 (30%) CR. At time of ibrutinib initiation, ten patients had active chronic GVHD that resolved under Ibrutinib, whilst a single patient developed limited de novo chronic GVHD on Ibrutinib. Fourteen patients discontinued ibrutinib, four because of toxicity and ten because of disease progression. Overall, 14 patients progressed (median PFS = 24 months) among them 10 died. Two-year OS and PFS probabilities were 72% (95% CI: 52-84) and 50% (95% CI: 32-66), respectively. Patients with late relapse after allo-HSCT (≥24 months) had a better PFS after ibrutinib. Our study shows that ibrutinib can be safely administered for CLL relapse after allo-HSCT, with comparable efficacy to non-transplanted patients with high-risk disease.
中文翻译:

依鲁替尼作为异基因HCT后的挽救疗法,用于治疗慢性淋巴细胞性白血病。
我们研究的目的是提供关于异位HSCT治疗CLL后依鲁替尼作为挽救治疗的安全性和有效性的信息。总共包括56位患者,其中36位(64%)为男性;移植时的中位年龄为48岁(范围:35-64岁),移植前治疗线的中位数量为3(1-10)。异基因造血干细胞移植和依鲁替尼之间的中位时间为30个月(范围:1-140)。总体上,有40(71%)位患者对依鲁替尼有反应;公关23(41%),CR 17(30%)。在开始依鲁替尼时,十名患者的活动性慢性GVHD在依鲁替尼的作用下消退,而一名患者在依鲁替尼上发展了有限的从头开始的慢性GVHD。十四名患者停用依鲁替尼,四名因毒性而停药,十名因疾病进展而停药。总体而言,有14例患者进展(中位PFS = 24个月),其中10例死亡。两年OS和PFS概率分别为72%(95%CI:52-84)和50%(95%CI:32-66)。异体造血干细胞移植(≥24个月)后晚期复发的患者在依鲁替尼治疗后有较好的PFS。我们的研究表明,异位HSCT后依鲁替尼可以安全地用于CLL复发,与非移植性高危疾病患者的疗效相当。
更新日期:2019-11-08
中文翻译:

依鲁替尼作为异基因HCT后的挽救疗法,用于治疗慢性淋巴细胞性白血病。
我们研究的目的是提供关于异位HSCT治疗CLL后依鲁替尼作为挽救治疗的安全性和有效性的信息。总共包括56位患者,其中36位(64%)为男性;移植时的中位年龄为48岁(范围:35-64岁),移植前治疗线的中位数量为3(1-10)。异基因造血干细胞移植和依鲁替尼之间的中位时间为30个月(范围:1-140)。总体上,有40(71%)位患者对依鲁替尼有反应;公关23(41%),CR 17(30%)。在开始依鲁替尼时,十名患者的活动性慢性GVHD在依鲁替尼的作用下消退,而一名患者在依鲁替尼上发展了有限的从头开始的慢性GVHD。十四名患者停用依鲁替尼,四名因毒性而停药,十名因疾病进展而停药。总体而言,有14例患者进展(中位PFS = 24个月),其中10例死亡。两年OS和PFS概率分别为72%(95%CI:52-84)和50%(95%CI:32-66)。异体造血干细胞移植(≥24个月)后晚期复发的患者在依鲁替尼治疗后有较好的PFS。我们的研究表明,异位HSCT后依鲁替尼可以安全地用于CLL复发,与非移植性高危疾病患者的疗效相当。











































 京公网安备 11010802027423号
京公网安备 11010802027423号