Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tau deletion reduces plaque-associated BACE1 accumulation and decelerates plaque formation in a mouse model of Alzheimer's disease.
The EMBO Journal ( IF 9.4 ) Pub Date : 2019-11-07 , DOI: 10.15252/embj.2019102345 Finn Peters 1, 2 , Hazal Salihoglu 1, 2 , Katrin Pratsch 1 , Etienne Herzog 3, 4 , Martina Pigoni 1, 2 , Carmelo Sgobio 1 , Stefan F Lichtenthaler 1, 2, 5 , Ulf Neumann 6 , Jochen Herms 1, 2, 7
The EMBO Journal ( IF 9.4 ) Pub Date : 2019-11-07 , DOI: 10.15252/embj.2019102345 Finn Peters 1, 2 , Hazal Salihoglu 1, 2 , Katrin Pratsch 1 , Etienne Herzog 3, 4 , Martina Pigoni 1, 2 , Carmelo Sgobio 1 , Stefan F Lichtenthaler 1, 2, 5 , Ulf Neumann 6 , Jochen Herms 1, 2, 7
Affiliation
|
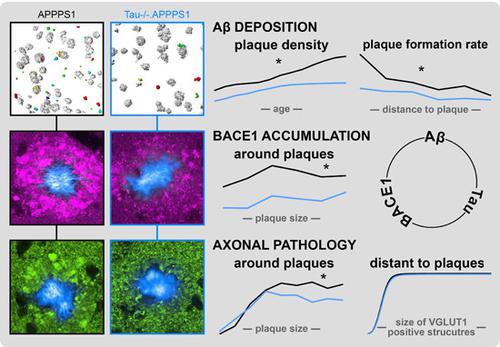
In Alzheimer's disease, BACE1 protease initiates the amyloidogenic processing of amyloid precursor protein (APP) that eventually results in synthesis of β-amyloid (Aβ) peptide. Aβ deposition in turn causes accumulation of BACE1 in plaque-associated dystrophic neurites, thereby potentiating progressive Aβ deposition once initiated. Since systemic pharmacological BACE inhibition causes adverse effects in humans, it is important to identify strategies that specifically normalize overt BACE1 activity around plaques. The microtubule-associated protein tau regulates axonal transport of proteins, and tau deletion rescues Aβ-induced transport deficits in vitro. In the current study, long-term in vivo two-photon microscopy and immunohistochemistry were performed in tau-deficient APPPS1 mice. Tau deletion reduced plaque-associated axonal pathology and BACE1 accumulation without affecting physiological BACE1 expression distant from plaques. Thereby, tau deletion effectively decelerated formation of new plaques and reduced plaque compactness. The data revealed that tau reinforces Aβ deposition, presumably by contributing to accumulation of BACE1 in plaque-associated dystrophies. Targeting tau-dependent mechanisms could become a suitable strategy to specifically reduce overt BACE1 activity around plaques, thereby avoiding adverse effects of systemic BACE inhibition.
中文翻译:

在阿尔茨海默病小鼠模型中,Tau 缺失可减少斑块相关的 BACE1 积累并减缓斑块形成。
在阿尔茨海默病中,BACE1 蛋白酶启动淀粉样前体蛋白 (APP) 的淀粉样蛋白形成过程,最终导致 β-淀粉样蛋白 (Aβ) 肽的合成。 Aβ 沉积反过来会导致 BACE1 在斑块相关的营养不良性神经突中积聚,从而一旦开始就会增强进行性 Aβ 沉积。由于全身药理学 BACE 抑制会对人类产生不良影响,因此确定专门使斑块周围明显的 BACE1 活性正常化的策略非常重要。微管相关蛋白 tau 调节蛋白质的轴突运输,tau 缺失可挽救 Aβ 诱导的体外运输缺陷。在目前的研究中,在 tau 缺陷 APPPS1 小鼠中进行了长期体内双光子显微镜和免疫组织化学分析。 Tau 缺失减少了斑块相关的轴突病理和 BACE1 积累,而不影响远离斑块的生理 BACE1 表达。因此,tau 缺失有效地减缓了新斑块的形成并降低了斑块的致密性。数据显示,tau 蛋白可能通过促进斑块相关营养不良中 BACE1 的积累而增强 Aβ 沉积。针对 tau 依赖性机制可能成为特异性降低斑块周围明显 BACE1 活性的合适策略,从而避免全身 BACE 抑制的不利影响。
更新日期:2019-12-02
中文翻译:

在阿尔茨海默病小鼠模型中,Tau 缺失可减少斑块相关的 BACE1 积累并减缓斑块形成。
在阿尔茨海默病中,BACE1 蛋白酶启动淀粉样前体蛋白 (APP) 的淀粉样蛋白形成过程,最终导致 β-淀粉样蛋白 (Aβ) 肽的合成。 Aβ 沉积反过来会导致 BACE1 在斑块相关的营养不良性神经突中积聚,从而一旦开始就会增强进行性 Aβ 沉积。由于全身药理学 BACE 抑制会对人类产生不良影响,因此确定专门使斑块周围明显的 BACE1 活性正常化的策略非常重要。微管相关蛋白 tau 调节蛋白质的轴突运输,tau 缺失可挽救 Aβ 诱导的体外运输缺陷。在目前的研究中,在 tau 缺陷 APPPS1 小鼠中进行了长期体内双光子显微镜和免疫组织化学分析。 Tau 缺失减少了斑块相关的轴突病理和 BACE1 积累,而不影响远离斑块的生理 BACE1 表达。因此,tau 缺失有效地减缓了新斑块的形成并降低了斑块的致密性。数据显示,tau 蛋白可能通过促进斑块相关营养不良中 BACE1 的积累而增强 Aβ 沉积。针对 tau 依赖性机制可能成为特异性降低斑块周围明显 BACE1 活性的合适策略,从而避免全身 BACE 抑制的不利影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号