当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Novel Disassembly Mechanisms of Sigmoid Aβ42 Protofibrils by Introduced Neutral and Charged Drug Molecules.
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2019-12-18 , DOI: 10.1021/acschemneuro.9b00550 Xiaofeng Xing 1 , Chengqiang Liu 1 , Aqsa Ali 1 , Baotao Kang 1 , Ping Li 2 , Hongqi Ai 1
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2019-12-18 , DOI: 10.1021/acschemneuro.9b00550 Xiaofeng Xing 1 , Chengqiang Liu 1 , Aqsa Ali 1 , Baotao Kang 1 , Ping Li 2 , Hongqi Ai 1
Affiliation
|
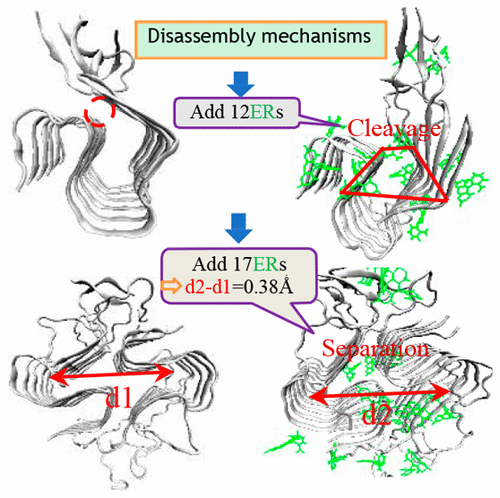
Alzheimer's disease (AD) is characterized by fibrillar deposits of amyloid-β (Aβ) peptides and neurofibrillary tangles of Tau proteins. Aβ peptides are composed of 37-49 residues, among which the Aβ42 isoform is particularly toxic and aggregation-prone and is enriched in the plaques of AD brains and thus considered central to the development of AD. Therefore, disaggregation and disruption provide potential therapeutic approaches to reduce, inhibit, and even reverse Aβ aggregation. Here we capture the atomic-level details of the interactions between sigmoid Aβ42 fibril 2MXU or 5KK3 and either natural tanshinone compounds TS1 or TS0 or negatively charged ER, proposing two unprecedented disassembly mechanisms. Natural TS1 or TS0 prefers to insert into the cavity together with part at the surface of the 2MXU to open up the mouth and twist the conformation, destroying the ordered growth of subsequent monomers along the fibril axis. For the more compact two-fold 5KK3 , attachment of TS1 or TS0 at the surface including some inserted in cavity results in the separation of the two folds. In the two sigmoid fibril systems, it is no longer applicable for the routine criteria to assess Aβ42 fibril disassembly by introduction of these drugs, such as either reduced H-bond number, decreased β-sheet contents, or both. ER, like-charged to Aβ42 fibril, is especially exceptional, and departs utterly from the neutral ones to disassemble Aβ42 fibril. Besides the inapplicable routine criteria, positive binding energy between ER and Aβ42 fibril also deviates from the hypotheses of "ligands exhibiting greater affinity for the β-amyloid peptide are effective at altering its aggregation and inhibiting cell toxicity" ( Cairo et al. , Biochemistry 2002 , 41 , 8620 - 8629 ) but results in stronger disassembly effect on the two kinds of sigmoid Aβ42 fibrils than neutral TS0 or TS1. The disassembly power of charged ER molecules derives from its stronger deformation ability to the conformation of Aβ42 fibril than the neutral ones, twisting the one-fold 2MXU into tapered-shape and separating two-fold 5KK3 in two parts further, which is in great agreement with experimental observations ( Irwin et al. Biomacromolecules 2013 , 14 ( 1 ), 264 - 274 ). The unusual disassembly mechanisms fill the gaps and offer an alternative direction in engineering new inhibitors to treat AD.
中文翻译:

中性和带电药物分子引入乙状结肠Aβ42原纤维的新机制。
阿尔茨海默氏病(AD)的特征在于淀粉样β(Aβ)肽的纤维状沉积和Tau蛋白的神经原纤维缠结。Aβ肽由37-49个残基组成,其中Aβ42同种型特别有毒且易于聚集,并富集在AD脑的斑块中,因此被认为是AD发育的关键。因此,分解和破坏提供了减少,抑制甚至逆转Aβ聚集的潜在治疗方法。在这里,我们捕获了乙状结肠Aβ42原纤维2MXU或5KK3与天然丹参酮化合物TS1或TS0或带负电的ER之间相互作用的原子级细节,提出了两种前所未有的拆卸机理。天然TS1或TS0倾向于将其与2MXU表面的一部分一起插入腔中,以张开嘴巴并扭曲构象,从而破坏后续单体沿原纤维轴的有序生长。对于更紧凑的两折5KK3,TS1或TS0在表面的附着(包括一些插入空腔中)会导致两折的分离。在两个乙状结肠原纤维系统中,不再适用于通过引入这些药物来评估Aβ42纤维原位分解的常规标准,例如降低的H键数,降低的β-折叠片含量或两者兼而有之。ER,与Aβ42的原纤维一样带电荷,特别例外,它完全脱离中性,从而分解Aβ42的原纤维。除了不适用的常规标准外,ER与Aβ42原纤维之间的正结合能也偏离了“对β-淀粉样肽表现出更大亲和力的配体在改变其聚集和抑制细胞毒性方面有效的假说”(Cairo et al。,Biochemistry 2002,41,8620-8629 ),但对两种乙状结肠Aβ42原纤维的分解作用要比中性TS0或TS1强。带电的ER分子的分解能力是由于其对Aβ42原纤维构象的变形能力强于中性,从而将单倍的2MXU扭曲成锥形,再将两倍的5KK3分成两部分,这是非常一致的具有实验观察性(Irwin等人,Biomacromolecules 2013,14(1),264-274)。
更新日期:2019-12-19
中文翻译:

中性和带电药物分子引入乙状结肠Aβ42原纤维的新机制。
阿尔茨海默氏病(AD)的特征在于淀粉样β(Aβ)肽的纤维状沉积和Tau蛋白的神经原纤维缠结。Aβ肽由37-49个残基组成,其中Aβ42同种型特别有毒且易于聚集,并富集在AD脑的斑块中,因此被认为是AD发育的关键。因此,分解和破坏提供了减少,抑制甚至逆转Aβ聚集的潜在治疗方法。在这里,我们捕获了乙状结肠Aβ42原纤维2MXU或5KK3与天然丹参酮化合物TS1或TS0或带负电的ER之间相互作用的原子级细节,提出了两种前所未有的拆卸机理。天然TS1或TS0倾向于将其与2MXU表面的一部分一起插入腔中,以张开嘴巴并扭曲构象,从而破坏后续单体沿原纤维轴的有序生长。对于更紧凑的两折5KK3,TS1或TS0在表面的附着(包括一些插入空腔中)会导致两折的分离。在两个乙状结肠原纤维系统中,不再适用于通过引入这些药物来评估Aβ42纤维原位分解的常规标准,例如降低的H键数,降低的β-折叠片含量或两者兼而有之。ER,与Aβ42的原纤维一样带电荷,特别例外,它完全脱离中性,从而分解Aβ42的原纤维。除了不适用的常规标准外,ER与Aβ42原纤维之间的正结合能也偏离了“对β-淀粉样肽表现出更大亲和力的配体在改变其聚集和抑制细胞毒性方面有效的假说”(Cairo et al。,Biochemistry 2002,41,8620-8629 ),但对两种乙状结肠Aβ42原纤维的分解作用要比中性TS0或TS1强。带电的ER分子的分解能力是由于其对Aβ42原纤维构象的变形能力强于中性,从而将单倍的2MXU扭曲成锥形,再将两倍的5KK3分成两部分,这是非常一致的具有实验观察性(Irwin等人,Biomacromolecules 2013,14(1),264-274)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号