当前位置:
X-MOL 学术
›
Inorg. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interaction of U(VI) with α-MnO2@layered double hydroxides by combined batch experiments and spectroscopy studies
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2019-11-07 , DOI: 10.1039/c9qi01316d Junping Ma 1, 2, 3, 4, 5 , Chen Wang 1, 2, 3, 4, 5 , Qiuyu Zhao 1, 2, 3, 4, 5 , Jianlin Ren 1, 2, 3, 4, 5 , Zhe Chen 1, 2, 3, 4, 5 , Jianjun Wang 1, 2, 3, 4, 5
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2019-11-07 , DOI: 10.1039/c9qi01316d Junping Ma 1, 2, 3, 4, 5 , Chen Wang 1, 2, 3, 4, 5 , Qiuyu Zhao 1, 2, 3, 4, 5 , Jianlin Ren 1, 2, 3, 4, 5 , Zhe Chen 1, 2, 3, 4, 5 , Jianjun Wang 1, 2, 3, 4, 5
Affiliation
|
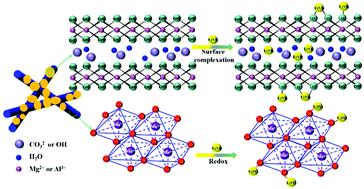
Uranium is of high concern in the field of environmental remediation because of its high fluidity, radioactivity, biological toxicity and long life. Removing U(VI) from wastewater is of great significance to both environment and biology. Herein, the composite adsorbent α-MnO2@LDHs composed of α-MnO2 and layered double hydroxides (LDHs) was constructed, and U(VI) adsorption experiments under different conditions were systematically carried out. The results manifested that the maximum U(VI) removal capacity of α-MnO2@LDHs was 135.52 mg g−1 at 298 K through the formation of inner-sphere surface complexes and redox reactions. In particular, at 328 K, the removal amount reached 564.97 mg g−1, which suggested the potential to treat high-temperature radioactive wastewater. Furthermore, α-MnO2@LDHs exhibited stability in a wide range of ionic strength (0.001–0.1 M) and pH (5.0–12.0), strong resistance to foreign ion interference, and rapid adsorption capacity. These made α-MnO2@LDHs an outstanding candidate for repair materials, and performed well even in simulated environments. In-depth and systematic spectra analysis revealed that the active functional groups were Al– and Mg–OH. Mn3+ and CO32− also made important contributions to the combination of U(VI). This work might promote the development of MnO2 combined with various metal LDHs, providing a reference for designing excellent repair materials.
中文翻译:

分批实验和光谱研究相结合的U(VI)与α-MnO2层状双氢氧化物的相互作用
铀由于其高流动性,放射性,生物毒性和长寿命而在环境修复领域受到高度关注。从废水中去除U(VI)对环境和生物学都具有重要意义。在此,复合吸附剂α-MnO的2个@LDHs由α-的MnO 2和层状双氢氧化物(水滑石)构建,和U(VI)在不同条件下吸附实验系统进行。结果表现的最大U(VI)去除α-的MnO容量2个@LDHs是135.52毫克克-1通过形成内球表面配合物和氧化还原反应在298 K时发生。尤其是在328 K时,去除量达到564.97 mg g -1,这表明有潜力处理高温放射性废水。此外,α-MnO的2 @LDHs在宽范围内的离子强度(0.001-0.1 M)和pH值(5.0-12.0),外国离子干扰能力强,快速的吸附能力表现出的稳定性。这些由α-MnO的2 @LDHs杰出的候选修补材料,并在模拟的环境中表现良好均匀。深入和系统的光谱分析表明,活性官能团为Al–和Mg–OH。Mn 3+和CO 3 2-也为U(VI)的组合做出了重要贡献。这项工作可能会促进MnO 2与各种金属LDH结合的发展,为设计优异的修复材料提供参考。
更新日期:2019-11-07
中文翻译:

分批实验和光谱研究相结合的U(VI)与α-MnO2层状双氢氧化物的相互作用
铀由于其高流动性,放射性,生物毒性和长寿命而在环境修复领域受到高度关注。从废水中去除U(VI)对环境和生物学都具有重要意义。在此,复合吸附剂α-MnO的2个@LDHs由α-的MnO 2和层状双氢氧化物(水滑石)构建,和U(VI)在不同条件下吸附实验系统进行。结果表现的最大U(VI)去除α-的MnO容量2个@LDHs是135.52毫克克-1通过形成内球表面配合物和氧化还原反应在298 K时发生。尤其是在328 K时,去除量达到564.97 mg g -1,这表明有潜力处理高温放射性废水。此外,α-MnO的2 @LDHs在宽范围内的离子强度(0.001-0.1 M)和pH值(5.0-12.0),外国离子干扰能力强,快速的吸附能力表现出的稳定性。这些由α-MnO的2 @LDHs杰出的候选修补材料,并在模拟的环境中表现良好均匀。深入和系统的光谱分析表明,活性官能团为Al–和Mg–OH。Mn 3+和CO 3 2-也为U(VI)的组合做出了重要贡献。这项工作可能会促进MnO 2与各种金属LDH结合的发展,为设计优异的修复材料提供参考。











































 京公网安备 11010802027423号
京公网安备 11010802027423号