当前位置:
X-MOL 学术
›
Beilstein. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Acid-catalyzed rearrangements in arenes: interconversions in the quaterphenyl series
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2019-11-06 , DOI: 10.3762/bjoc.15.258 Sarah L Skraba-Joiner , Carter J Holt , Richard P Johnson
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2019-11-06 , DOI: 10.3762/bjoc.15.258 Sarah L Skraba-Joiner , Carter J Holt , Richard P Johnson
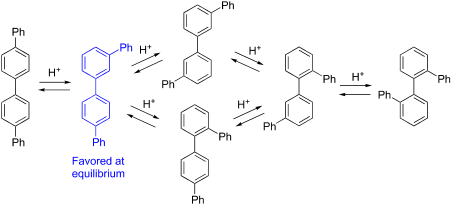
|
Arenes undergo rearrangement of phenyl, alkyl, halogen and other groups through the intermediacy of ipso arenium ions in which a proton is attached at the same carbon as the migrating substituent. Interconversions among the six quaterphenyl isomers have been studied here as a model for rearrangements of linear polyphenyls. All reactions were carried out in 1 M CF3SO3H (TfOH) in dichloroethane at 150 °C in a microwave reactor for 30–60 min, with product formation assessed by high field NMR analysis. Under these reaction conditions, m,p'-quaterphenyl is the equilibrium product. This isomer is unchanged by the reaction conditions and all other quaterphenyl isomers rearrange to m,p' as the dominant or sole product. DFT computations with inclusion of implicit solvation support a complex network of phenyl and biphenyl shifts, with barriers to rearrangement in the range of 10–21 kcal/mol. Consistent with experiments, the lowest energy arenium ion located on this surface is due to protonation of m,p'-quaterphenyl. This supports thermodynamic control based on carbocation energies.
中文翻译:

芳烃中的酸催化重排:四苯基系列的相互转化
芳烃通过中间性经历的苯基,烷基,卤素和其它基团的重排的本位arenium其中质子连接在相同碳作为迁移取代基的离子。此处已研究了六个四苯基异构体之间的相互转化,作为线性聚苯基重排的模型。所有反应均在微波反应器中于150°C的二氯乙烷中的1 M CF 3 SO 3 H(TfOH)中进行30-60分钟,并通过高场NMR分析评估产物的形成。在这些反应条件下,m,p′-四苯基为平衡产物。该异构体在反应条件下保持不变,所有其他四苯基异构体均重排为m,p'作为主导产品或唯一产品。包含隐含溶剂化的DFT计算支持复杂的苯基和联苯转移网络,重排障碍在10–21 kcal / mol的范围内。与实验一致,位于该表面上的最低能量的芳族离子是由于m,p'-四苯基的质子化所致。这支持了基于碳正离子能量的热力学控制。
更新日期:2019-11-06
中文翻译:

芳烃中的酸催化重排:四苯基系列的相互转化
芳烃通过中间性经历的苯基,烷基,卤素和其它基团的重排的本位arenium其中质子连接在相同碳作为迁移取代基的离子。此处已研究了六个四苯基异构体之间的相互转化,作为线性聚苯基重排的模型。所有反应均在微波反应器中于150°C的二氯乙烷中的1 M CF 3 SO 3 H(TfOH)中进行30-60分钟,并通过高场NMR分析评估产物的形成。在这些反应条件下,m,p′-四苯基为平衡产物。该异构体在反应条件下保持不变,所有其他四苯基异构体均重排为m,p'作为主导产品或唯一产品。包含隐含溶剂化的DFT计算支持复杂的苯基和联苯转移网络,重排障碍在10–21 kcal / mol的范围内。与实验一致,位于该表面上的最低能量的芳族离子是由于m,p'-四苯基的质子化所致。这支持了基于碳正离子能量的热力学控制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号