当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthetic Oligosaccharide-Based Vaccines Protect Mice from Clostridioides difficile Infections.
ACS Chemical Biology ( IF 4 ) Pub Date : 2019-11-19 , DOI: 10.1021/acschembio.9b00642 Felix Broecker 1, 2 , Erik Wegner 3 , Bruna M S Seco 1, 2 , Paulina Kaplonek 1, 2 , Maria Bräutigam 1, 2 , Armin Ensser 4 , Frederick Pfister 5 , Christoph Daniel 5 , Christopher E Martin 1, 2 , Jochen Mattner 3 , Peter H Seeberger 1, 2
ACS Chemical Biology ( IF 4 ) Pub Date : 2019-11-19 , DOI: 10.1021/acschembio.9b00642 Felix Broecker 1, 2 , Erik Wegner 3 , Bruna M S Seco 1, 2 , Paulina Kaplonek 1, 2 , Maria Bräutigam 1, 2 , Armin Ensser 4 , Frederick Pfister 5 , Christoph Daniel 5 , Christopher E Martin 1, 2 , Jochen Mattner 3 , Peter H Seeberger 1, 2
Affiliation
|
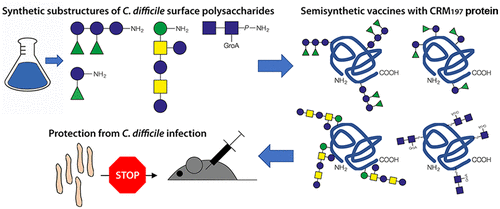
Infections with Clostridioides difficile (formerly Clostridium difficile) have increased in incidence, morbidity, and mortality over the past decade. Preventing infections is becoming increasingly important, as frontline antibiotics become less effective and frequently induce recurrence by disrupting intestinal microbiota. The clinically most advanced vaccine approaches prevent symptoms once C. difficile infection is established by inducing immunity to secreted clostridial cytotoxins. However, they do not inhibit bacterial colonization and thereby favor asymptomatic carriage. Synthetic oligosaccharides resembling the C. difficile surface glycans PS-I, PS-II, and PS-III are immunogenic and serve as basis for colonization-preventing vaccines. Here, we demonstrate that glycoconjugate vaccine candidates based on synthetic oligosaccharides protected mice from infections with two different C. difficile strains. Four synthetic antigens, ranging in size from disaccharides to hexasaccharides, were conjugated to CRM197, which is a carrier protein used in commercial vaccines. The vaccine candidates induced glycan-specific antibodies in mice and substantially limited C. difficile colonization and colitis after experimental infection. The glycoconjugates ameliorated intestinal pathology more substantially than a toxin-targeting vaccine. Colonization of the gut by C. difficile was selectively inhibited while intestinal microbiota remained preserved. Passive transfer experiments with anti-PS-I serum revealed that protection is mediated by specific antiglycan antibodies; however, cell-mediated immunity likely also contributed to protection in vivo. Thus, glycoconjugate vaccines against C. difficile are a complementary approach to toxin-targeting strategies and are advancing through preclinical work.
中文翻译:

基于合成寡糖的疫苗可保护小鼠免受艰难梭菌感染。
在过去十年中,艰难梭菌(以前称为艰难梭菌)感染的发病率,发病率和死亡率都有所增加。预防感染变得越来越重要,因为一线抗生素的有效性降低,并经常通过破坏肠道菌群来诱导复发。一旦通过诱导对分泌的梭菌细胞毒素的免疫力建立了艰难梭菌感染,临床上最先进的疫苗方法就可以预防症状。但是,它们不抑制细菌定植,因此有利于无症状携带。类似于艰难梭菌表面聚糖PS-1,PS-II和PS-III的合成寡糖具有免疫原性,可作为预防定植疫苗的基础。这里,我们证明基于合成寡糖的糖缀合物疫苗候选物可保护小鼠免受两种不同艰难梭菌菌株的感染。从二糖到六糖大小不等的四种合成抗原与CRM197偶联,后者是商业疫苗中使用的载体蛋白。候选疫苗在小鼠中诱导了聚糖特异性抗体,并在实验感染后大大限制了艰难梭菌的定殖和结肠炎。糖缀合物比靶向毒素的疫苗更能显着改善肠道病理。艰难梭菌对肠道的定殖被选择性抑制,而肠道菌群则得以保留。用抗PS-1血清进行的被动转移实验表明,保护作用是由特定的抗聚糖抗体介导的。然而,细胞介导的免疫力也可能有助于体内保护。因此,针对艰难梭菌的糖缀合物疫苗是毒素靶向策略的一种补充方法,并且正在通过临床前研究而不断发展。
更新日期:2019-11-19
中文翻译:

基于合成寡糖的疫苗可保护小鼠免受艰难梭菌感染。
在过去十年中,艰难梭菌(以前称为艰难梭菌)感染的发病率,发病率和死亡率都有所增加。预防感染变得越来越重要,因为一线抗生素的有效性降低,并经常通过破坏肠道菌群来诱导复发。一旦通过诱导对分泌的梭菌细胞毒素的免疫力建立了艰难梭菌感染,临床上最先进的疫苗方法就可以预防症状。但是,它们不抑制细菌定植,因此有利于无症状携带。类似于艰难梭菌表面聚糖PS-1,PS-II和PS-III的合成寡糖具有免疫原性,可作为预防定植疫苗的基础。这里,我们证明基于合成寡糖的糖缀合物疫苗候选物可保护小鼠免受两种不同艰难梭菌菌株的感染。从二糖到六糖大小不等的四种合成抗原与CRM197偶联,后者是商业疫苗中使用的载体蛋白。候选疫苗在小鼠中诱导了聚糖特异性抗体,并在实验感染后大大限制了艰难梭菌的定殖和结肠炎。糖缀合物比靶向毒素的疫苗更能显着改善肠道病理。艰难梭菌对肠道的定殖被选择性抑制,而肠道菌群则得以保留。用抗PS-1血清进行的被动转移实验表明,保护作用是由特定的抗聚糖抗体介导的。然而,细胞介导的免疫力也可能有助于体内保护。因此,针对艰难梭菌的糖缀合物疫苗是毒素靶向策略的一种补充方法,并且正在通过临床前研究而不断发展。



























 京公网安备 11010802027423号
京公网安备 11010802027423号