当前位置:
X-MOL 学术
›
J. Mol. Recognit.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Binding mechanism of maltol with catalase investigated by spectroscopy, molecular docking, and enzyme activity assay.
Journal of Molecular Recognition ( IF 2.7 ) Pub Date : 2019-11-06 , DOI: 10.1002/jmr.2822 Mengling Huo 1 , Lining Zhao 1 , Ting Wang 2 , Wansong Zong 3 , Rutao Liu 1
Journal of Molecular Recognition ( IF 2.7 ) Pub Date : 2019-11-06 , DOI: 10.1002/jmr.2822 Mengling Huo 1 , Lining Zhao 1 , Ting Wang 2 , Wansong Zong 3 , Rutao Liu 1
Affiliation
|
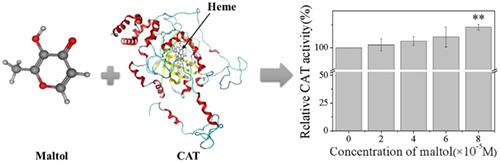
Maltol is a flavor additive that is widely used in the daily diet of humans, and its biosafety attention is concomitantly increasing. Catalase (CAT) is an antioxidant enzyme to maintain homeostasis in the tissue's environment of human body and protect cells from oxidative damages. The adverse effects of maltol to CAT activity within mouse hepatocytes as well as the structural and functional changes of CAT on molecular level were investigated by multiple spectroscopy techniques, enzyme activity experiments, and molecular docking. Results suggested that when the maltol concentrations reached to 8 × 10-5 mol L-1 , the viability of hepatocytes decreased to 93%, and CAT activity was stimulated by maltol to 111% than the control group after exposure for 24 hours. Changes in CAT activity on molecular level were consistent with those on cellular level. The fluorescence quenching of CAT by maltol was static with the forming of maltol-CAT complex. Moreover, ultraviolet-visible (UV-visible) absorption, synchronous fluorescence, and circular dichroism (CD) spectra reflected that the presence of maltol caused conformational change of CAT and made the CAT molecule skeleton loose and increased α-helix of CAT. Maltol mainly bound with CAT through hydrogen bond, and binding site that is near the heme ring in the enzyme activity center did not interact with its main amino acid residues. This study explores the combination between maltol and CAT, providing references for evaluating health damages caused by maltol.
中文翻译:

麦芽酚与过氧化氢酶的结合机理已通过光谱,分子对接和酶活性测定进行了研究。
麦芽酚是一种调味添加剂,已广泛用于人类的日常饮食中,因此其对生物安全性的关注也在不断增加。过氧化氢酶(CAT)是一种抗氧化酶,可在人体组织环境中维持体内平衡,并保护细胞免受氧化损伤。通过多种光谱技术,酶活性实验和分子对接研究了麦芽酚对小鼠肝细胞内CAT活性的不利影响以及CAT的结构和功能变化对分子水平的影响。结果表明,暴露24小时后,当麦芽酚的浓度达到8×10-5 mol L-1时,肝细胞的活力降低至93%,麦芽酚刺激CAT的活性达到对照组的111%。CAT活性在分子水平上的变化与细胞水平上的变化一致。麦芽酚对CAT的荧光猝灭是静态的,形成了麦芽酚-CAT复合物。此外,紫外-可见(UV-可见)吸收,同步荧光和圆二色性(CD)光谱反映出麦芽酚的存在引起CAT的构象变化,并使CAT分子骨架疏松并增加了CAT的α-螺旋。麦芽酚主要通过氢键与CAT结合,在酶活性中心血红素环附近的结合位点不与其主要氨基酸残基相互作用。这项研究探索了麦芽酚和CAT之间的结合,为评估由麦芽酚引起的健康损害提供了参考。圆二色性(CD)光谱表明,麦芽酚的存在引起CAT的构象变化,使CAT分子骨架松散并增加了CAT的α-螺旋。麦芽酚主要通过氢键与CAT结合,在酶活性中心血红素环附近的结合位点不与其主要氨基酸残基相互作用。这项研究探索了麦芽酚和CAT之间的结合,为评估由麦芽酚引起的健康损害提供了参考。圆二色性(CD)光谱表明,麦芽酚的存在引起CAT的构象变化,使CAT分子骨架松散并增加了CAT的α-螺旋。麦芽酚主要通过氢键与CAT结合,在酶活性中心血红素环附近的结合位点不与其主要氨基酸残基相互作用。这项研究探索了麦芽酚和CAT之间的结合,为评估由麦芽酚引起的健康损害提供了参考。
更新日期:2020-02-04
中文翻译:

麦芽酚与过氧化氢酶的结合机理已通过光谱,分子对接和酶活性测定进行了研究。
麦芽酚是一种调味添加剂,已广泛用于人类的日常饮食中,因此其对生物安全性的关注也在不断增加。过氧化氢酶(CAT)是一种抗氧化酶,可在人体组织环境中维持体内平衡,并保护细胞免受氧化损伤。通过多种光谱技术,酶活性实验和分子对接研究了麦芽酚对小鼠肝细胞内CAT活性的不利影响以及CAT的结构和功能变化对分子水平的影响。结果表明,暴露24小时后,当麦芽酚的浓度达到8×10-5 mol L-1时,肝细胞的活力降低至93%,麦芽酚刺激CAT的活性达到对照组的111%。CAT活性在分子水平上的变化与细胞水平上的变化一致。麦芽酚对CAT的荧光猝灭是静态的,形成了麦芽酚-CAT复合物。此外,紫外-可见(UV-可见)吸收,同步荧光和圆二色性(CD)光谱反映出麦芽酚的存在引起CAT的构象变化,并使CAT分子骨架疏松并增加了CAT的α-螺旋。麦芽酚主要通过氢键与CAT结合,在酶活性中心血红素环附近的结合位点不与其主要氨基酸残基相互作用。这项研究探索了麦芽酚和CAT之间的结合,为评估由麦芽酚引起的健康损害提供了参考。圆二色性(CD)光谱表明,麦芽酚的存在引起CAT的构象变化,使CAT分子骨架松散并增加了CAT的α-螺旋。麦芽酚主要通过氢键与CAT结合,在酶活性中心血红素环附近的结合位点不与其主要氨基酸残基相互作用。这项研究探索了麦芽酚和CAT之间的结合,为评估由麦芽酚引起的健康损害提供了参考。圆二色性(CD)光谱表明,麦芽酚的存在引起CAT的构象变化,使CAT分子骨架松散并增加了CAT的α-螺旋。麦芽酚主要通过氢键与CAT结合,在酶活性中心血红素环附近的结合位点不与其主要氨基酸残基相互作用。这项研究探索了麦芽酚和CAT之间的结合,为评估由麦芽酚引起的健康损害提供了参考。



























 京公网安备 11010802027423号
京公网安备 11010802027423号