当前位置:
X-MOL 学术
›
Eur. J. Pharm. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Investigation of drug-polymer miscibility, biorelevant dissolution, and bioavailability improvement of Dolutegravir-polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol graft copolymer solid dispersions.
European Journal of Pharmaceutical Sciences ( IF 4.3 ) Pub Date : 2019-11-06 , DOI: 10.1016/j.ejps.2019.105137 Dani Lakshman 1 , Mohith Chegireddy 1 , Geeta K Hanegave 1 , K Navya Sree 1 , Naveen Kumar 2 , Shaila A Lewis 3 , Swapnil J Dengale 1
European Journal of Pharmaceutical Sciences ( IF 4.3 ) Pub Date : 2019-11-06 , DOI: 10.1016/j.ejps.2019.105137 Dani Lakshman 1 , Mohith Chegireddy 1 , Geeta K Hanegave 1 , K Navya Sree 1 , Naveen Kumar 2 , Shaila A Lewis 3 , Swapnil J Dengale 1
Affiliation
|
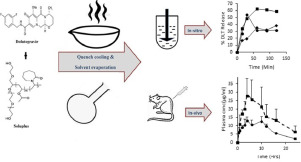
The aim of the current study was to prepare the efficacious amorphous solid dispersion of poorly water-soluble compound, Dolutegravir. After theoretical and experimental determination of drug-polymer miscibility, polyvinyl caprolactam-polyvinyl acetate-polyethylne glycol graft copolymer was chosen as a polymer. The solid dispersions of Dolutegravir were prepared by quench cooling and solvent evaporation method. Though quench cooling successfully stabilized the drug into amorphous form, solvent evaporation technique failed to render the drug completely amorphous. Owing to the negative Gibbs free energy at room temperature, the prepared dispersions were found stable at room temperature for 60 days. To resolve the overlapping contribution of micellar solubilization and amorphicity in improving the dissolution characteristics of Dolutegravir, the in vitro dissolution studies were performed in USP phosphate buffer as well as bio-relevant media. The dissolution advantage between prepared dispersions and pure drug in USP phosphate buffer was found bridged in the bio-relevant media. For this, the micellar solubilization driven dissolution of Dolutegravir in the presence of bile and lecithin micelles was thought as a contributing factor. Nevertheless, the dissolution advantage of dispersions prepared by quench cooling method was found endured in FeSSIF, which was thought to be due to its amorphicity leading to molecular level dissolution. Subsequently, the dissolution advantage was translated into the improved flux. Further, in vivo oral bioavailability was investigated for the dispersion prepared by quench cooling by using crystalline Dolutegravir as a control. The overall exposure of Dolutegravir was improved by 1.7 fold (AUC), while the maximum plasma concentration (Cmax) demonstrated 2 fold increase after comparing with crystalline Dolutegravir.
中文翻译:

Dolutegravir-聚乙烯己内酰胺-聚乙酸乙烯酯-聚乙二醇接枝共聚物固体分散体的药物-聚合物混溶性,生物相关溶出度和生物利用度提高的研究。
当前研究的目的是制备水溶性差的化合物Dolutegravir的有效无定形固体分散体。在理论上和实验上确定了药物与聚合物的混溶性之后,选择了聚乙烯基己内酰胺-乙酸乙烯酯-聚乙二醇接枝共聚物作为聚合物。通过骤冷和溶剂蒸发法制备Dolutegravir的固体分散体。尽管淬火冷却成功地将药物稳定为无定形形式,但溶剂蒸发技术未能使药物完全变为无定形。由于室温下负的吉布斯自由能,发现制备的分散体在室温下稳定60天。为了解决胶束增溶作用和非晶性在改善Dolutegravir溶出度特性方面的重叠贡献,在USP磷酸盐缓冲液以及生物相关介质中进行了体外溶出度研究。发现在生物相关介质中弥合了制备的分散体和纯药物在USP磷酸盐缓冲液之间的溶解优势。为此,在胆汁和卵磷脂胶束的存在下,胶束增溶作用驱使Dolutegravir溶解是一个促成因素。然而,发现通过淬火冷却法制备的分散体在FeSSIF中具有溶解优势,这被认为是由于其无定形性导致分子水平的溶解。随后,溶解优势转化为改进的通量。此外,研究了通过使用结晶Dolutegravir作为对照通过骤冷冷却制备的分散体的体内口服生物利用度。
更新日期:2019-11-06
中文翻译:

Dolutegravir-聚乙烯己内酰胺-聚乙酸乙烯酯-聚乙二醇接枝共聚物固体分散体的药物-聚合物混溶性,生物相关溶出度和生物利用度提高的研究。
当前研究的目的是制备水溶性差的化合物Dolutegravir的有效无定形固体分散体。在理论上和实验上确定了药物与聚合物的混溶性之后,选择了聚乙烯基己内酰胺-乙酸乙烯酯-聚乙二醇接枝共聚物作为聚合物。通过骤冷和溶剂蒸发法制备Dolutegravir的固体分散体。尽管淬火冷却成功地将药物稳定为无定形形式,但溶剂蒸发技术未能使药物完全变为无定形。由于室温下负的吉布斯自由能,发现制备的分散体在室温下稳定60天。为了解决胶束增溶作用和非晶性在改善Dolutegravir溶出度特性方面的重叠贡献,在USP磷酸盐缓冲液以及生物相关介质中进行了体外溶出度研究。发现在生物相关介质中弥合了制备的分散体和纯药物在USP磷酸盐缓冲液之间的溶解优势。为此,在胆汁和卵磷脂胶束的存在下,胶束增溶作用驱使Dolutegravir溶解是一个促成因素。然而,发现通过淬火冷却法制备的分散体在FeSSIF中具有溶解优势,这被认为是由于其无定形性导致分子水平的溶解。随后,溶解优势转化为改进的通量。此外,研究了通过使用结晶Dolutegravir作为对照通过骤冷冷却制备的分散体的体内口服生物利用度。











































 京公网安备 11010802027423号
京公网安备 11010802027423号