当前位置:
X-MOL 学术
›
Nat. Struct. Mol. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
UPR proteins IRE1 and PERK switch BiP from chaperone to ER stress sensor.
Nature Structural & Molecular Biology ( IF 16.8 ) Pub Date : 2019-11-06 , DOI: 10.1038/s41594-019-0324-9 Megan C Kopp 1 , Natacha Larburu 1 , Vinoth Durairaj 1 , Christopher J Adams 1 , Maruf M U Ali 1
Nature Structural & Molecular Biology ( IF 16.8 ) Pub Date : 2019-11-06 , DOI: 10.1038/s41594-019-0324-9 Megan C Kopp 1 , Natacha Larburu 1 , Vinoth Durairaj 1 , Christopher J Adams 1 , Maruf M U Ali 1
Affiliation
|
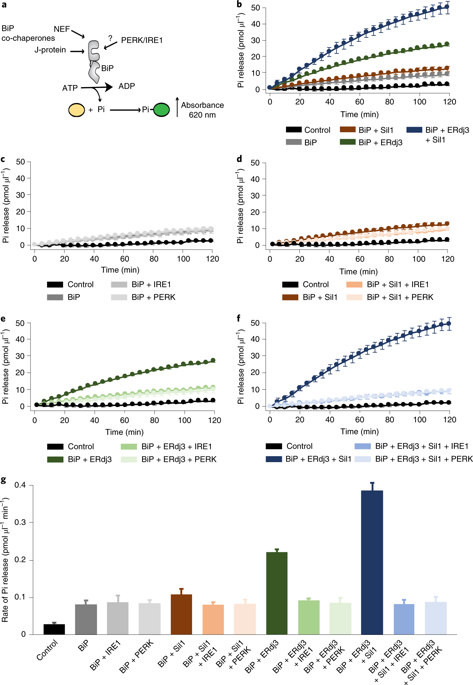
BiP is a major endoplasmic reticulum (ER) chaperone and is suggested to act as primary sensor in the activation of the unfolded protein response (UPR). How BiP operates as a molecular chaperone and as an ER stress sensor is unknown. Here, by reconstituting components of human UPR, ER stress and BiP chaperone systems, we discover that the interaction of BiP with the luminal domains of UPR proteins IRE1 and PERK switch BiP from its chaperone cycle into an ER stress sensor cycle by preventing the binding of its co-chaperones, with loss of ATPase stimulation. Furthermore, misfolded protein-dependent dissociation of BiP from IRE1 is primed by ATP but not ADP. Our data elucidate a previously unidentified mechanistic cycle of BiP function that explains its ability to act as an Hsp70 chaperone and ER stress sensor.
中文翻译:

UPR 蛋白 IRE1 和 PERK 将 BiP 从伴侣转换为 ER 压力传感器。
BiP 是一种主要的内质网 (ER) 伴侣,被建议作为激活未折叠蛋白反应 (UPR) 的主要传感器。BiP 如何作为分子伴侣和 ER 压力传感器运作尚不清楚。在这里,通过重组人类 UPR、ER 应激和 BiP 伴侣系统的成分,我们发现 BiP 与 UPR 蛋白 IRE1 和 PERK 的腔结构域的相互作用通过阻止 BIP 的结合将 BiP 从其伴侣循环转换为 ER 应激传感器循环。它的共同伴侣,失去了 ATP 酶的刺激。此外,BiP 与 IRE1 的错误折叠蛋白依赖性解离是由 ATP 而不是 ADP 引发的。我们的数据阐明了以前未知的 BiP 功能机械循环,解释了它作为 Hsp70 伴侣和 ER 压力传感器的能力。
更新日期:2019-11-06
中文翻译:

UPR 蛋白 IRE1 和 PERK 将 BiP 从伴侣转换为 ER 压力传感器。
BiP 是一种主要的内质网 (ER) 伴侣,被建议作为激活未折叠蛋白反应 (UPR) 的主要传感器。BiP 如何作为分子伴侣和 ER 压力传感器运作尚不清楚。在这里,通过重组人类 UPR、ER 应激和 BiP 伴侣系统的成分,我们发现 BiP 与 UPR 蛋白 IRE1 和 PERK 的腔结构域的相互作用通过阻止 BIP 的结合将 BiP 从其伴侣循环转换为 ER 应激传感器循环。它的共同伴侣,失去了 ATP 酶的刺激。此外,BiP 与 IRE1 的错误折叠蛋白依赖性解离是由 ATP 而不是 ADP 引发的。我们的数据阐明了以前未知的 BiP 功能机械循环,解释了它作为 Hsp70 伴侣和 ER 压力传感器的能力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号