当前位置:
X-MOL 学术
›
JAMA Dermatol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficacy and Safety of Dupilumab in Adolescents With Uncontrolled Moderate to Severe Atopic Dermatitis: A Phase 3 Randomized Clinical Trial.
JAMA Dermatology ( IF 11.5 ) Pub Date : 2019-11-06 , DOI: 10.1001/jamadermatol.2019.3336 Eric L Simpson 1 , Amy S Paller 2, 3 , Elaine C Siegfried 4 , Mark Boguniewicz 5 , Lawrence Sher 6 , Melinda J Gooderham 7, 8, 9 , Lisa A Beck 10 , Emma Guttman-Yassky 11, 12, 13 , David Pariser 14 , Andrew Blauvelt 15 , Jamie Weisman 16 , Benjamin Lockshin 17, 18 , Thomas Hultsch 19 , Qin Zhang 20 , Mohamed A Kamal 20 , John D Davis 20 , Bolanle Akinlade 20 , Heribert Staudinger 21 , Jennifer D Hamilton 20 , Neil M H Graham 20 , Gianluca Pirozzi 21 , Abhijit Gadkari 20 , Laurent Eckert 22 , Neil Stahl 20 , George D Yancopoulos 20 , Marcella Ruddy 20 , Ashish Bansal 20
JAMA Dermatology ( IF 11.5 ) Pub Date : 2019-11-06 , DOI: 10.1001/jamadermatol.2019.3336 Eric L Simpson 1 , Amy S Paller 2, 3 , Elaine C Siegfried 4 , Mark Boguniewicz 5 , Lawrence Sher 6 , Melinda J Gooderham 7, 8, 9 , Lisa A Beck 10 , Emma Guttman-Yassky 11, 12, 13 , David Pariser 14 , Andrew Blauvelt 15 , Jamie Weisman 16 , Benjamin Lockshin 17, 18 , Thomas Hultsch 19 , Qin Zhang 20 , Mohamed A Kamal 20 , John D Davis 20 , Bolanle Akinlade 20 , Heribert Staudinger 21 , Jennifer D Hamilton 20 , Neil M H Graham 20 , Gianluca Pirozzi 21 , Abhijit Gadkari 20 , Laurent Eckert 22 , Neil Stahl 20 , George D Yancopoulos 20 , Marcella Ruddy 20 , Ashish Bansal 20
Affiliation
|
Importance
Adolescents with atopic dermatitis (AD) have high disease burden negatively affecting quality of life, with limited treatment options. The efficacy and safety of dupilumab, a monoclonal antibody, approved for treatment in adolescent patients with inadequately controlled AD, remain unknown in this patient population.
Objective
To assess the efficacy and safety of dupilumab monotherapy in adolescents with moderate to severe inadequately controlled AD.
Design, Setting, and Participants
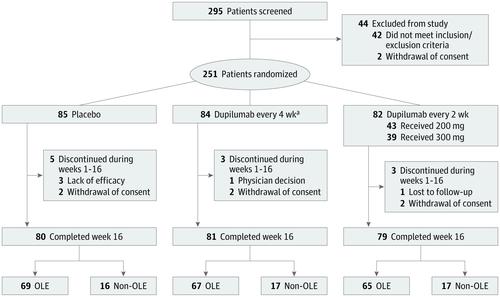
A randomized, double-blind, parallel-group, phase 3 clinical trial was conducted at 45 US and Canadian centers between March 21, 2017, and June 5, 2018. A total of 251 adolescents with moderate to severe AD inadequately controlled by topical medications or for whom topical therapy was inadvisable were included.
Interventions
Patients were randomized (1:1:1; interactive-response system; stratified by severity and body weight) to 16-week treatment with dupilumab, 200 mg (n = 43; baseline weight <60 kg), or dupilumab, 300 mg (n = 39; baseline weight ≥60 kg), every 2 weeks; dupilumab, 300 mg, every 4 weeks (n = 84); or placebo (n = 85).
Main Outcomes and Measures
Proportion of patients with 75% or more improvement from baseline in Eczema Area and Severity Index (EASI-75) (scores range from 0 to 72, with higher scores indicating greater severity) and Investigator's Global Assessment (IGA) 0 or 1 on a 5-point scale (scores range from 0 to 4, with higher scores indicating greater severity) at week 16.
Results
A total of 251 patients were randomized (mean [SD] age, 14.5 [1.7] years; 148 [59.0%] male). Of 250 patients with data available on concurrent allergic conditions, most had comorbid type 2 diseases (asthma, 134 [53.6%]; food allergies, 60.8%; allergic rhinitis, 65.6%). A total of 240 patients (95.6%) completed the study. Dupilumab achieved both coprimary end points at week 16. The proportion of patients with EASI-75 improvement from baseline increased (every 2 weeks, 41.5%; every 4 weeks, 38.1%; placebo, 8.2%) with differences vs placebo of 33.2% (95% CI, 21.1%-45.4%) for every 2 weeks and 29.9% (95% CI, 17.9%-41.8%) for every 4 weeks (P < .001). Efficacy of the every-2-week regimen was generally superior to the every-4-week regimen. Patients in the dupilumab arms had higher percentage values of conjunctivitis (every 2 weeks, 9.8%; every 4 weeks, 10.8%; placebo, 4.7%) and injection-site reactions (every 2 weeks, 8.5%; every 4 weeks, 6.0%; placebo, 3.5%), and lower nonherpetic skin infections (every 2 weeks, 9.8%; every 4 weeks, 9.6%; placebo, 18.8%).
Conclusions and Relevance
In this study, dupilumab significantly improved AD signs, symptoms, and quality of life in adolescents with moderate to severe AD, with an acceptable safety profile. Placebo-corrected efficacy and safety of dupilumab were similar in adolescents and adults.
Trial Registration
ClinicalTrials.gov identifier: NCT03054428.
中文翻译:

Dupilumab 对患有不受控制的中度至重度特应性皮炎的青少年的疗效和安全性:一项 3 期随机临床试验。
重要性 患有特应性皮炎 (AD) 的青少年疾病负担较高,对生活质量产生负面影响,且治疗选择有限。 dupilumab 是一种单克隆抗体,已被批准用于治疗未得到充分控制的 AD 青少年患者,但该患者群体的疗效和安全性仍不清楚。目的 评估 dupilumab 单药治疗中度至重度未得到充分控制的 AD 青少年的疗效和安全性。设计、设置和参与者 2017年3月21日至2018年6月5日期间,在美国和加拿大的45个中心进行了一项随机、双盲、平行组、3期临床试验。共有251名患有中度至重度疾病的青少年局部药物不能充分控制 AD 或局部治疗不宜的患者也包括在内。干预措施 患者被随机分配(1:1:1;交互式反应系统;按严重程度和体重分层)接受 16 周的 dupilumab 200 mg 治疗(n = 43;基线体重 <60 kg)或 dupilumab 300 mg (n = 39;基线体重≥60 kg),每 2 周一次; dupilumab,300 mg,每 4 周一次 (n = 84);或安慰剂 (n = 85)。主要结果和措施 湿疹面积和严重程度指数 (EASI-75)(分数范围为 0 至 72,分数越高表明严重程度越高)和研究者总体评估 (IGA) 较基线改善 75% 或以上的患者比例 0 或第 16 周时,按 5 分制评分为 1 分(分数范围为 0 至 4,分数越高表明严重程度越高)。 结果 共有 251 名患者被随机分组(平均 [SD] 年龄,14.5 [1.7] 岁;148 [59.0] 岁) %] 男性)。在 250 名有并发过敏性疾病数据的患者中,大多数患有 2 型疾病(哮喘,134 例 [53.6%];食物过敏,60.8%;过敏性鼻炎,65.6%)。 共有 240 名患者 (95.6%) 完成了研究。 Dupilumab 在第 16 周达到了两个共同主要终点。 EASI-75 较基线改善的患者比例增加(每 2 周,41.5%;每 4 周,38.1%;安慰剂,8.2%),与安慰剂相比差异为 33.2%(每 2 周为 95% CI,21.1%-45.4%),每 4 周为 29.9% (95% CI,17.9%-41.8%) (P < .001)。每 2 周一次的治疗方案的疗效通常优于每 4 周一次的治疗方案。 dupilumab 组患者的结膜炎百分比(每 2 周,9.8%;每 4 周,10.8%;安慰剂,4.7%)和注射部位反应(每 2 周,8.5%;每 4 周,6.0%)百分比较高;安慰剂,3.5%),并降低非疱疹皮肤感染(每 2 周一次,9.8%;每 4 周一次,9.6%;安慰剂,18.8%)。结论和相关性 在这项研究中,dupilumab 显着改善了中度至重度 AD 青少年的 AD 体征、症状和生活质量,且安全性可接受。 Dupilumab 的安慰剂校正疗效和安全性在青少年和成人中相似。试验注册 ClinicalTrials.gov 标识符:NCT03054428。
更新日期:2020-01-08
中文翻译:

Dupilumab 对患有不受控制的中度至重度特应性皮炎的青少年的疗效和安全性:一项 3 期随机临床试验。
重要性 患有特应性皮炎 (AD) 的青少年疾病负担较高,对生活质量产生负面影响,且治疗选择有限。 dupilumab 是一种单克隆抗体,已被批准用于治疗未得到充分控制的 AD 青少年患者,但该患者群体的疗效和安全性仍不清楚。目的 评估 dupilumab 单药治疗中度至重度未得到充分控制的 AD 青少年的疗效和安全性。设计、设置和参与者 2017年3月21日至2018年6月5日期间,在美国和加拿大的45个中心进行了一项随机、双盲、平行组、3期临床试验。共有251名患有中度至重度疾病的青少年局部药物不能充分控制 AD 或局部治疗不宜的患者也包括在内。干预措施 患者被随机分配(1:1:1;交互式反应系统;按严重程度和体重分层)接受 16 周的 dupilumab 200 mg 治疗(n = 43;基线体重 <60 kg)或 dupilumab 300 mg (n = 39;基线体重≥60 kg),每 2 周一次; dupilumab,300 mg,每 4 周一次 (n = 84);或安慰剂 (n = 85)。主要结果和措施 湿疹面积和严重程度指数 (EASI-75)(分数范围为 0 至 72,分数越高表明严重程度越高)和研究者总体评估 (IGA) 较基线改善 75% 或以上的患者比例 0 或第 16 周时,按 5 分制评分为 1 分(分数范围为 0 至 4,分数越高表明严重程度越高)。 结果 共有 251 名患者被随机分组(平均 [SD] 年龄,14.5 [1.7] 岁;148 [59.0] 岁) %] 男性)。在 250 名有并发过敏性疾病数据的患者中,大多数患有 2 型疾病(哮喘,134 例 [53.6%];食物过敏,60.8%;过敏性鼻炎,65.6%)。 共有 240 名患者 (95.6%) 完成了研究。 Dupilumab 在第 16 周达到了两个共同主要终点。 EASI-75 较基线改善的患者比例增加(每 2 周,41.5%;每 4 周,38.1%;安慰剂,8.2%),与安慰剂相比差异为 33.2%(每 2 周为 95% CI,21.1%-45.4%),每 4 周为 29.9% (95% CI,17.9%-41.8%) (P < .001)。每 2 周一次的治疗方案的疗效通常优于每 4 周一次的治疗方案。 dupilumab 组患者的结膜炎百分比(每 2 周,9.8%;每 4 周,10.8%;安慰剂,4.7%)和注射部位反应(每 2 周,8.5%;每 4 周,6.0%)百分比较高;安慰剂,3.5%),并降低非疱疹皮肤感染(每 2 周一次,9.8%;每 4 周一次,9.6%;安慰剂,18.8%)。结论和相关性 在这项研究中,dupilumab 显着改善了中度至重度 AD 青少年的 AD 体征、症状和生活质量,且安全性可接受。 Dupilumab 的安慰剂校正疗效和安全性在青少年和成人中相似。试验注册 ClinicalTrials.gov 标识符:NCT03054428。











































 京公网安备 11010802027423号
京公网安备 11010802027423号