当前位置:
X-MOL 学术
›
Drug Test. Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A simple method to immunopurify erthyropoiesis stimulating agents from urine, aiming to optimize erythropoietin screening by SAR-PAGE.
Drug Testing and Analysis ( IF 2.6 ) Pub Date : 2019-12-30 , DOI: 10.1002/dta.2730 Carmel E Heiland 1 , Michèle Masquelier 1 , Hasanuzzaman Bhuiyan 1 , Magnus Ericsson 1, 2
Drug Testing and Analysis ( IF 2.6 ) Pub Date : 2019-12-30 , DOI: 10.1002/dta.2730 Carmel E Heiland 1 , Michèle Masquelier 1 , Hasanuzzaman Bhuiyan 1 , Magnus Ericsson 1, 2
Affiliation
|
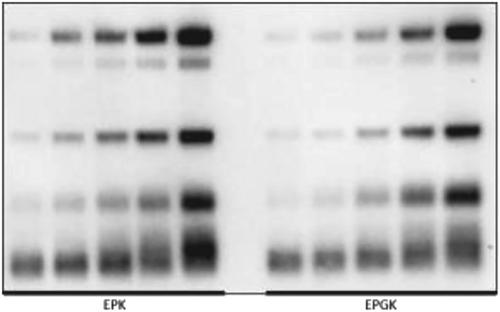
The efficiency of the immunopurification step of urinary erythropoietin (EPO) and recombinant forms is important for their optimal detection in antidoping screening. Previous investigations of immunopurification techniques have been done for immunomagnetic beads, EPO Purification Kit (EPK) columns (MAIIA Diagnostics), and enzyme‐linked immunosorbent assay (ELISA) microplates (Stemcell Technologies) conjugated/coated with anti‐EPO antibodies. In this study, a new immunopurification technique using anti‐EPO sepharose gel beads, developed by MAIIA Diagnostics, to simplify and minimize sample handling was evaluated. This EPO Purification Gel Kit (EPGK) was compared with our current routine EPK for limit of detection (LOD). Linearity, recovery, repeatability, sample incubation time, and sample volume were also evaluated for EPGK. The LODs and linearity for EPK and EPGK were comparable to each other and the recovery for BRP, NESP, CERA, and EPO‐Fc were within the range of other studies, and concentration of the sample eluate improved the recovery results. Little variation was seen within days, between days, and between operators. A 90 minute incubation of the sample with the sepharose gel beads is sufficient for most of the erythropoiesis stimulating agents (ESAs) tested, with 10 mL being an optimal sample volume for EPGK. The improved sample handling, higher sample throughput and the reduced working time demonstrate that the EPGK is a better alternative to the current MAIIA EPK immunopurification method for urine. The EPO Purification Gel Kit (from MAIIA Diagnostics) was evaluated and validated for immunopurification of endogenous erythropoietin and exogenous erythropoiesis stimulating agents from urine samples. The kit was a better alternative to that currently used (EPO Purification Kit) in many antidoping laboratories because it improves sample handling and increases sample throughput.
中文翻译:

一种从尿液中免疫纯化促红细胞生成素刺激剂的简单方法,旨在通过SAR-PAGE优化促红细胞生成素的筛选。
尿促红细胞生成素(EPO)和重组形式的免疫纯化步骤的效率对其在反兴奋剂筛选中的最佳检测非常重要。以前已经对免疫磁珠,EPO纯化试剂盒(EPK)柱(MAIIA Diagnostics)和缀有抗EPO抗体的酶联免疫吸附测定(ELISA)微孔板(Stemcell Technologies)进行了免疫纯化技术的研究。在这项研究中,评估了一种由MAIIA Diagnostics开发的使用抗EPO琼脂糖凝胶珠的新型免疫纯化技术,以简化和最小化样品处理。将此EPO纯化凝胶试剂盒(EPGK)与我们目前的常规EPK进行了检测限(LOD)的比较。还针对EPGK评估了线性,回收率,可重复性,样品孵育时间和样品量。EPK和EPGK的LOD和线性相互可比,BRP,NESP,CERA和EPO-Fc的回收率在其他研究范围之内,样品洗脱液的浓度提高了回收率。几天之内,几天之间以及操作员之间几乎看不到变化。将样品与琼脂糖凝胶珠孵育90分钟就足以测试大多数促红细胞生成刺激剂(ESA),其中10 mL是EPGK的最佳样品量。改进的样品处理,更高的样品通量和减少的工作时间证明,EPGK是当前MAIIA EPK尿液免疫纯化方法的更好替代品。评估并验证了EPO纯化凝胶试剂盒(来自MAIIA Diagnostics)用于尿液样本中的内源性促红细胞生成素和外源性促红细胞生成素的免疫纯化。该试剂盒是许多反掺杂实验室中目前使用的试剂盒(EPO纯化试剂盒)的更好替代品,因为它可以改善样品处理并增加样品通量。
更新日期:2019-12-30
中文翻译:

一种从尿液中免疫纯化促红细胞生成素刺激剂的简单方法,旨在通过SAR-PAGE优化促红细胞生成素的筛选。
尿促红细胞生成素(EPO)和重组形式的免疫纯化步骤的效率对其在反兴奋剂筛选中的最佳检测非常重要。以前已经对免疫磁珠,EPO纯化试剂盒(EPK)柱(MAIIA Diagnostics)和缀有抗EPO抗体的酶联免疫吸附测定(ELISA)微孔板(Stemcell Technologies)进行了免疫纯化技术的研究。在这项研究中,评估了一种由MAIIA Diagnostics开发的使用抗EPO琼脂糖凝胶珠的新型免疫纯化技术,以简化和最小化样品处理。将此EPO纯化凝胶试剂盒(EPGK)与我们目前的常规EPK进行了检测限(LOD)的比较。还针对EPGK评估了线性,回收率,可重复性,样品孵育时间和样品量。EPK和EPGK的LOD和线性相互可比,BRP,NESP,CERA和EPO-Fc的回收率在其他研究范围之内,样品洗脱液的浓度提高了回收率。几天之内,几天之间以及操作员之间几乎看不到变化。将样品与琼脂糖凝胶珠孵育90分钟就足以测试大多数促红细胞生成刺激剂(ESA),其中10 mL是EPGK的最佳样品量。改进的样品处理,更高的样品通量和减少的工作时间证明,EPGK是当前MAIIA EPK尿液免疫纯化方法的更好替代品。评估并验证了EPO纯化凝胶试剂盒(来自MAIIA Diagnostics)用于尿液样本中的内源性促红细胞生成素和外源性促红细胞生成素的免疫纯化。该试剂盒是许多反掺杂实验室中目前使用的试剂盒(EPO纯化试剂盒)的更好替代品,因为它可以改善样品处理并增加样品通量。











































 京公网安备 11010802027423号
京公网安备 11010802027423号