Oncogenesis ( IF 5.9 ) Pub Date : 2019-11-06 , DOI: 10.1038/s41389-019-0174-7 Zilong Li , Jun Xia , Mingming Fang , Yong Xu
|
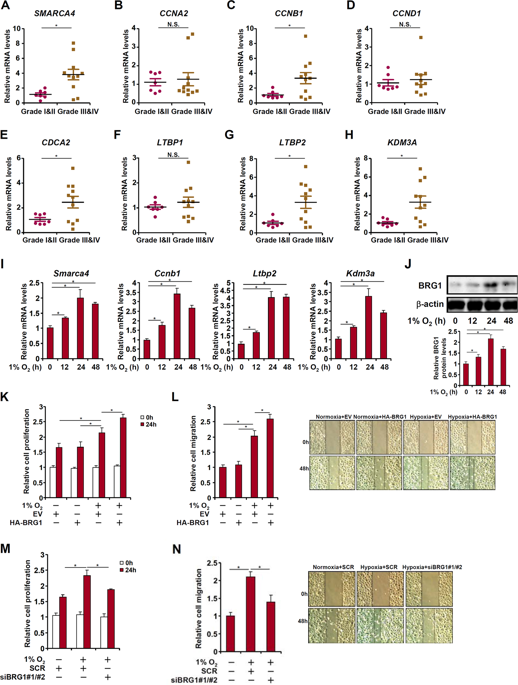
Malignant lung cancer cells are characterized by uncontrolled proliferation and migration. Aberrant lung cancer cell proliferation and migration are programmed by altered cancer transcriptome. The underlying epigenetic mechanism is unclear. Here we report that expression levels of BRG1, a chromatin remodeling protein, were significantly up-regulated in human lung cancer biopsy specimens of higher malignancy grades compared to those of lower grades. Small interfering RNA mediated depletion or pharmaceutical inhibition of BRG1 suppressed proliferation and migration of lung cancer cells. BRG1 depletion or inhibition was paralleled by down-regulation of cyclin B1 (CCNB1) and latent TGF-β binding protein 2 (LTBP2) in lung cancer cells. Further analysis revealed that BRG1 directly bound to the CCNB1 promoter to activate transcription in response to hypoxia stimulation by interacting with E2F1. On the other hand, BRG1 interacted with Sp1 to activate LTBP2 transcription. Mechanistically, BRG1 regulated CCNB1 and LTBP2 transcription by altering histone modifications on target promoters. Specifically, BRG1 recruited KDM3A, a histone H3K9 demethylase, to remove dimethyl H3K9 from target gene promoters thereby activating transcription. KDM3A knockdown achieved equivalent effects as BRG1 silencing by diminishing lung cancer proliferation and migration. Of interest, BRG1 directly activated KDM3A transcription by forming a complex with HIF-1α. In conclusion, our data unveil a novel epigenetic mechanism whereby malignant lung cancer cells acquired heightened ability to proliferate and migrate. Targeting BRG1 may yield effective interventional strategies against malignant lung cancers.
中文翻译:

染色质重塑蛋白BRG1对肺癌细胞增殖和迁移的表观遗传调控
恶性肺癌细胞的特征在于不受控制的增殖和迁移。异常的肺癌细胞增殖和迁移是通过改变癌症转录组来编程的。潜在的表观遗传机制尚不清楚。在这里,我们报道在高恶性等级的人肺癌活检标本中,BRG1(一种染色质重塑蛋白)的表达水平显着高于低恶性等级的人肺癌活检标本。小干扰RNA介导的耗竭或BRG1的药物抑制抑制了肺癌细胞的增殖和迁移。在肺癌细胞中,BRG1的消耗或抑制与细胞周期蛋白B1(CCNB1)和潜在的TGF-β结合蛋白2(LTBP2)的下调平行。进一步的分析表明,BRG1直接与CCNB1启动子结合,通过与E2F1相互作用来响应缺氧刺激而激活转录。另一方面,BRG1与Sp1相互作用以激活LTBP2转录。从机制上讲,BRG1通过改变靶启动子上的组蛋白修饰来调控CCNB1和LTBP2转录。具体而言,BRG1募集了组蛋白H3K9脱甲基酶KDM3A,以从目标基因启动子中去除二甲基H3K9,从而激活转录。通过减少肺癌的增殖和迁移,KDM3A敲低可达到与BRG1沉默相同的效果。令人感兴趣的是,BRG1通过与HIF-1α形成复合物而直接激活KDM3A转录。综上所述,我们的数据揭示了一种新颖的表观遗传机制,由此恶性肺癌细胞获得了增强的增殖和迁移能力。靶向BRG1可能产生针对恶性肺癌的有效干预策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号