Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Caspofungin vs Fluconazole Prophylaxis on Invasive Fungal Disease Among Children and Young Adults With Acute Myeloid Leukemia
JAMA ( IF 63.1 ) Pub Date : 2019-11-05 , DOI: 10.1001/jama.2019.15702 Brian T Fisher 1 , Theoklis Zaoutis 1 , Christopher C Dvorak 2 , Michael Nieder 3 , Danielle Zerr 4 , John R Wingard 5 , Colleen Callahan 6 , Doojduen Villaluna 7 , Lu Chen 8 , Ha Dang 9 , Adam J Esbenshade 10 , Sarah Alexander 11 , Joseph M Wiley 12 , Lillian Sung 11
JAMA ( IF 63.1 ) Pub Date : 2019-11-05 , DOI: 10.1001/jama.2019.15702 Brian T Fisher 1 , Theoklis Zaoutis 1 , Christopher C Dvorak 2 , Michael Nieder 3 , Danielle Zerr 4 , John R Wingard 5 , Colleen Callahan 6 , Doojduen Villaluna 7 , Lu Chen 8 , Ha Dang 9 , Adam J Esbenshade 10 , Sarah Alexander 11 , Joseph M Wiley 12 , Lillian Sung 11
Affiliation
|
Importance
Children, adolescents, and young adults with acute myeloid leukemia are at high risk of life-threatening invasive fungal disease with both yeasts and molds. Objective
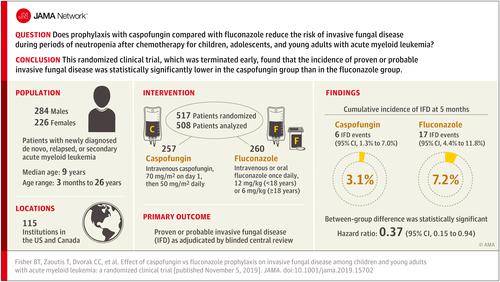
To compare the efficacy of caspofungin vs fluconazole prophylaxis against proven or probable invasive fungal disease and invasive aspergillosis during neutropenia following acute myeloid leukemia chemotherapy. Design, Setting, and Participants
This multicenter, randomized, open-label, clinical trial enrolled patients aged 3 months to 30 years with newly diagnosed de novo, relapsed, or secondary acute myeloid leukemia being treated at 115 US and Canadian institutions (April 2011-November 2016; last follow-up June 30, 2018). Interventions
Participants were randomly assigned during the first chemotherapy cycle to prophylaxis with caspofungin (n = 257) or fluconazole (n = 260). Prophylaxis was administered during the neutropenic period following each chemotherapy cycle. Main Outcomes and Measures
The primary outcome was proven or probable invasive fungal disease as adjudicated by blinded central review. Secondary outcomes were invasive aspergillosis, empirical antifungal therapy, and overall survival. Results
The second interim efficacy analysis and an unplanned futility analysis based on 394 patients appeared to have suggested futility, so the study was closed to accrual. Among the 517 participants who were randomized (median age, 9 years [range, 0-26 years]; 44% female), 508 (98%) completed the trial. The 23 proven or probable invasive fungal disease events (6 caspofungin vs 17 fluconazole) included 14 molds, 7 yeasts, and 2 fungi not further categorized. The 5-month cumulative incidence of proven or probable invasive fungal disease was 3.1% (95% CI, 1.3%-7.0%) in the caspofungin group vs 7.2% (95% CI, 4.4%-11.8%) in the fluconazole group (overall P = .03 by log-rank test) and for cumulative incidence of proven or probable invasive aspergillosis was 0.5% (95% CI, 0.1%-3.5%) with caspofungin vs 3.1% (95% CI, 1.4%-6.9%) with fluconazole (overall P = .046 by log-rank test). No statistically significant differences in empirical antifungal therapy (71.9% caspofungin vs 69.5% fluconazole, overall P = .78 by log-rank test) or 2-year overall survival (68.8% caspofungin vs 70.8% fluconazole, overall P = .66 by log-rank test) were observed. The most common toxicities were hypokalemia (22 caspofungin vs 13 fluconazole), respiratory failure (6 caspofungin vs 9 fluconazole), and elevated alanine transaminase (4 caspofungin vs 8 fluconazole). Conclusions and Relevance
Among children, adolescents, and young adults with acute myeloid leukemia, prophylaxis with caspofungin compared with fluconazole resulted in significantly lower incidence of invasive fungal disease. The findings suggest that caspofungin may be considered for prophylaxis against invasive fungal disease, although study interpretation is limited by early termination due to an unplanned interim analysis that appeared to have suggested futility. Trial Registration
ClinicalTrials.gov Identifier: NCT01307579.
中文翻译:

卡泊芬净与氟康唑预防儿童和青年急性髓性白血病侵袭性真菌病的效果
重要性 患有急性髓细胞性白血病的儿童、青少年和年轻人患上酵母菌和霉菌侵袭性真菌病的危及生命的风险很高。目的 比较卡泊芬净与氟康唑预防急性髓系白血病化疗后中性粒细胞减少期间已证实或可能的侵袭性真菌病和侵袭性曲霉菌病的疗效。设计、设置和参与者 这项多中心、随机、开放标签的临床试验招募了 3 个月至 30 岁的患者,这些患者在美国和加拿大的 115 家机构接受治疗(2011 年 4 月- 2016 年 11 月;最后一次随访 2018 年 6 月 30 日)。干预 在第一个化疗周期内,参与者被随机分配到卡泊芬净 (n = 257) 或氟康唑 (n = 260) 进行预防。在每个化疗周期后的中性粒细胞减少期进行预防。主要结果和测量 主要结果是由盲法中央审查裁定的证实或可能的侵袭性真菌病。次要结果是侵袭性曲霉菌病、经验性抗真菌治疗和总生存期。结果 第二次中期疗效分析和基于 394 名患者的计划外无效分析似乎表明无效,因此该研究停止招募。在随机分配的 517 名参与者(中位年龄,9 岁 [范围,0-26 岁];44% 为女性)中,508 名 (98%) 完成了试验。23 种已证实或可能的侵袭性真菌病事件(6 例卡泊芬净 vs 17 例氟康唑)包括 14 种霉菌、7 种酵母菌和 2 种未进一步分类的真菌。卡泊芬净组证实或可能的侵袭性真菌病的 5 个月累积发生率为 3.1%(95% CI,1.3%-7.0%),而氟康唑组为 7.2%(95% CI,4.4%-11.8%)。总体 P = 0.03,通过对数秩检验)和已证实或可能的侵袭性曲霉菌病的累积发生率为 0.5%(95% CI,0.1%-3.5%),卡泊芬净为 3.1%(95% CI,1.4%-6.9%) ) 与氟康唑(通过对数秩检验,总体 P = .046)。经验性抗真菌治疗(卡泊芬净 71.9% vs 氟康唑 69.5%,对数秩检验总体 P = .78)或 2 年总生存率(卡泊芬净 68.8% vs 氟康唑 70.8%,对数 P = .66)无统计学差异-秩检验)进行了观察。最常见的毒性是低钾血症(卡泊芬净 22 对氟康唑 13)、呼吸衰竭(卡泊芬净 6 对氟康唑 9)、和丙氨酸转氨酶升高(4 个卡泊芬净对 8 个氟康唑)。结论和相关性 在患有急性髓性白血病的儿童、青少年和年轻成人中,与氟康唑相比,卡泊芬净预防显着降低了侵袭性真菌病的发生率。研究结果表明,卡泊芬净可被考虑用于预防侵袭性真菌病,尽管由于一项计划外的中期分析似乎表明无效,研究解释受到提前终止的限制。试验注册 ClinicalTrials.gov 标识符:NCT01307579。研究结果表明,卡泊芬净可被考虑用于预防侵袭性真菌病,尽管由于一项计划外的中期分析似乎表明无效,研究解释受到提前终止的限制。试验注册 ClinicalTrials.gov 标识符:NCT01307579。研究结果表明,卡泊芬净可被考虑用于预防侵袭性真菌病,尽管由于一项计划外的中期分析似乎表明无效,研究解释受到提前终止的限制。试验注册 ClinicalTrials.gov 标识符:NCT01307579。
更新日期:2019-11-05
中文翻译:

卡泊芬净与氟康唑预防儿童和青年急性髓性白血病侵袭性真菌病的效果
重要性 患有急性髓细胞性白血病的儿童、青少年和年轻人患上酵母菌和霉菌侵袭性真菌病的危及生命的风险很高。目的 比较卡泊芬净与氟康唑预防急性髓系白血病化疗后中性粒细胞减少期间已证实或可能的侵袭性真菌病和侵袭性曲霉菌病的疗效。设计、设置和参与者 这项多中心、随机、开放标签的临床试验招募了 3 个月至 30 岁的患者,这些患者在美国和加拿大的 115 家机构接受治疗(2011 年 4 月- 2016 年 11 月;最后一次随访 2018 年 6 月 30 日)。干预 在第一个化疗周期内,参与者被随机分配到卡泊芬净 (n = 257) 或氟康唑 (n = 260) 进行预防。在每个化疗周期后的中性粒细胞减少期进行预防。主要结果和测量 主要结果是由盲法中央审查裁定的证实或可能的侵袭性真菌病。次要结果是侵袭性曲霉菌病、经验性抗真菌治疗和总生存期。结果 第二次中期疗效分析和基于 394 名患者的计划外无效分析似乎表明无效,因此该研究停止招募。在随机分配的 517 名参与者(中位年龄,9 岁 [范围,0-26 岁];44% 为女性)中,508 名 (98%) 完成了试验。23 种已证实或可能的侵袭性真菌病事件(6 例卡泊芬净 vs 17 例氟康唑)包括 14 种霉菌、7 种酵母菌和 2 种未进一步分类的真菌。卡泊芬净组证实或可能的侵袭性真菌病的 5 个月累积发生率为 3.1%(95% CI,1.3%-7.0%),而氟康唑组为 7.2%(95% CI,4.4%-11.8%)。总体 P = 0.03,通过对数秩检验)和已证实或可能的侵袭性曲霉菌病的累积发生率为 0.5%(95% CI,0.1%-3.5%),卡泊芬净为 3.1%(95% CI,1.4%-6.9%) ) 与氟康唑(通过对数秩检验,总体 P = .046)。经验性抗真菌治疗(卡泊芬净 71.9% vs 氟康唑 69.5%,对数秩检验总体 P = .78)或 2 年总生存率(卡泊芬净 68.8% vs 氟康唑 70.8%,对数 P = .66)无统计学差异-秩检验)进行了观察。最常见的毒性是低钾血症(卡泊芬净 22 对氟康唑 13)、呼吸衰竭(卡泊芬净 6 对氟康唑 9)、和丙氨酸转氨酶升高(4 个卡泊芬净对 8 个氟康唑)。结论和相关性 在患有急性髓性白血病的儿童、青少年和年轻成人中,与氟康唑相比,卡泊芬净预防显着降低了侵袭性真菌病的发生率。研究结果表明,卡泊芬净可被考虑用于预防侵袭性真菌病,尽管由于一项计划外的中期分析似乎表明无效,研究解释受到提前终止的限制。试验注册 ClinicalTrials.gov 标识符:NCT01307579。研究结果表明,卡泊芬净可被考虑用于预防侵袭性真菌病,尽管由于一项计划外的中期分析似乎表明无效,研究解释受到提前终止的限制。试验注册 ClinicalTrials.gov 标识符:NCT01307579。研究结果表明,卡泊芬净可被考虑用于预防侵袭性真菌病,尽管由于一项计划外的中期分析似乎表明无效,研究解释受到提前终止的限制。试验注册 ClinicalTrials.gov 标识符:NCT01307579。









































 京公网安备 11010802027423号
京公网安备 11010802027423号