当前位置:
X-MOL 学术
›
Eur. J. Pharm. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Inhaled rapamycin solid lipid nano particles for the treatment of Lymphangioleiomyomatosis.
European Journal of Pharmaceutical Sciences ( IF 4.3 ) Pub Date : 2019-11-05 , DOI: 10.1016/j.ejps.2019.105098 Emelie Landh 1 , Lyn M Moir 1 , Larissa Gomes Dos Reis 2 , Daniela Traini 1 , Paul M Young 1 , Hui Xin Ong 1
European Journal of Pharmaceutical Sciences ( IF 4.3 ) Pub Date : 2019-11-05 , DOI: 10.1016/j.ejps.2019.105098 Emelie Landh 1 , Lyn M Moir 1 , Larissa Gomes Dos Reis 2 , Daniela Traini 1 , Paul M Young 1 , Hui Xin Ong 1
Affiliation
|
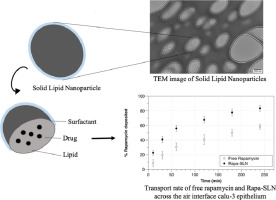
Lymphangioleiomyomatosis (LAM) is a rare lung disease characterized by uncontrolled growth of smooth muscle -like cells in the lungs that can spread via the lymphatic system to other parts of the body. The current treatment for LAM, oral rapamycin, is limited by its low oral bioavailability and side effects. This study aims to develop an inhaled formulation of rapamycin solid lipid nanoparticles (Rapa-SLNs) to avoid first-pass metabolism, increase invivo half-life and facilitate entry into the lymphatic system through the lungs. Rapa-SLNs were manufactured using a hot evaporation technique and freeze-dried overnight with 5% (w/v) mannitol and before being assessed further for particle characteristics and in vitro aerosol performance and release. The formulation's ability to penetrate through bronchial epithelial layer was evaluated using a Calu-3 cell model, while its ability to interfere with the LAM intracellular cascade was evaluated using Mouse Embryonic fibroblast (MEF) cells deficient for the tuberous sclerosis complex 2 (TSC2) and compared with rapamycin solution. Results showed that the Rapa- SLNs had the appropriate size (237.5 ± 1.8 nm), charge (-11.2), in vitro aerosol performance (MMAD=5.4 ± 0.4 μm) and sustained release profile suitable for entry into the lymphatic system via the pulmonary route. Additionally, the nanoparticles were transported at a faster rate across the bronchial epithelial layer compared to free rapamycin solution. The formulation also showed similar mTOR (mammalian target of Rapamycin) inhibition properties compared to free rapamycin, and was able to significantly decrease the amount of proliferation in TSC2 negative MEF cells. This formulation is therefore a promising alternative treatment for LAM patients, as it could potentially reduce problems associated with low bioavailability and side effects of current oral treatment.
中文翻译:

吸入雷帕霉素固体脂质纳米颗粒治疗淋巴管平滑肌瘤病。
淋巴管平滑肌肌瘤病(LAM)是一种罕见的肺部疾病,其特征是肺中平滑肌样细胞的不受控制的生长,这些细胞可以通过淋巴系统扩散到身体的其他部位。目前,LAM的口服雷帕霉素治疗方法受到口服生物利用度低和副作用的限制。这项研究旨在开发雷帕霉素固体脂质纳米颗粒(Rapa-SLNs)的吸入制剂,以避免首过代谢,增加体内半衰期并促进通过肺进入淋巴系统。Rapa-SLN使用热蒸发技术制造,并用5%(w / v)甘露醇冷冻干燥过夜,然后进一步评估颗粒特性以及体外气雾剂性能和释放。配方' 使用Calu-3细胞模型评估其穿透支气管上皮层的能力,而使用缺乏结节性硬化复合物2(TSC2)的小鼠胚胎成纤维细胞(MEF)细胞评估其对LAM细胞内级联的干扰能力,并进行比较与雷帕霉素溶液。结果表明,Rapa-SLNs具有合适的大小(237.5±1.8 nm),电荷(-11.2),体外气溶胶性能(MMAD = 5.4±0.4μm)和适合于通过肺进入淋巴系统的缓释曲线路线。另外,与游离雷帕霉素溶液相比,纳米颗粒以更快的速率跨支气管上皮层运输。与游离雷帕霉素相比,该制剂还显示出相似的mTOR(雷帕霉素的哺乳动物靶标)抑制特性,并能够显着降低TSC2阴性MEF细胞的增殖量。因此,该制剂对于LAM患者是有希望的替代治疗,因为它可以潜在地减少与当前口服治疗的低生物利用度和副作用相关的问题。
更新日期:2019-11-05
中文翻译:

吸入雷帕霉素固体脂质纳米颗粒治疗淋巴管平滑肌瘤病。
淋巴管平滑肌肌瘤病(LAM)是一种罕见的肺部疾病,其特征是肺中平滑肌样细胞的不受控制的生长,这些细胞可以通过淋巴系统扩散到身体的其他部位。目前,LAM的口服雷帕霉素治疗方法受到口服生物利用度低和副作用的限制。这项研究旨在开发雷帕霉素固体脂质纳米颗粒(Rapa-SLNs)的吸入制剂,以避免首过代谢,增加体内半衰期并促进通过肺进入淋巴系统。Rapa-SLN使用热蒸发技术制造,并用5%(w / v)甘露醇冷冻干燥过夜,然后进一步评估颗粒特性以及体外气雾剂性能和释放。配方' 使用Calu-3细胞模型评估其穿透支气管上皮层的能力,而使用缺乏结节性硬化复合物2(TSC2)的小鼠胚胎成纤维细胞(MEF)细胞评估其对LAM细胞内级联的干扰能力,并进行比较与雷帕霉素溶液。结果表明,Rapa-SLNs具有合适的大小(237.5±1.8 nm),电荷(-11.2),体外气溶胶性能(MMAD = 5.4±0.4μm)和适合于通过肺进入淋巴系统的缓释曲线路线。另外,与游离雷帕霉素溶液相比,纳米颗粒以更快的速率跨支气管上皮层运输。与游离雷帕霉素相比,该制剂还显示出相似的mTOR(雷帕霉素的哺乳动物靶标)抑制特性,并能够显着降低TSC2阴性MEF细胞的增殖量。因此,该制剂对于LAM患者是有希望的替代治疗,因为它可以潜在地减少与当前口服治疗的低生物利用度和副作用相关的问题。











































 京公网安备 11010802027423号
京公网安备 11010802027423号