Computational and Theoretical Chemistry ( IF 3.0 ) Pub Date : 2019-11-05 , DOI: 10.1016/j.comptc.2019.112643 Anindita Pati , T.K. Kundu , Snehanshu Pal
|
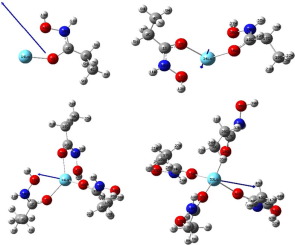
In this paper, Density Functional Method at B3LYP/SDD level has been implemented in order to study enhanced interaction with lanthanum ion (La3+) in presence of multiple molecules of chelating ligand hydroxyamide (HA) for efficient extraction of lanthanides. Geometrical analysis of optimized structure, thermochemical analysis, potential energy surface study, electron density profile determination and localized orbital locator analysis, molecular orbital analysis, density of states analysis and vibrational spectral (IR spectra) analysis of the complex consisting of one, two, three, and four hydroxyamide ligands with lanthanum have been performed. Calculated geometrical parameters, interaction energies, change in Gibb’s free energy, change in enthalpy, change in entropy, and HOMO-LUMO study indicate the formation feasibility, as well as stability of the complexes, is enhanced while a higher number of hydroxyamide ligand interact with La(III). Structural analysis and vibrational analysis exhibit the formation of hydrogen bond-like non-bonded interaction between hydroxyamide and lanthanum. The orbital analysis provides the underlying reason for the bond formation between lanthanum and doubly bonded oxygen atom with carbon in the hydroxyamide ligand. The above study helps to analyse the interaction of lanthanum with hydroxyamide in order to design chelating extractant for efficient extraction of this rare earth element.
中文翻译:

基于量子化学计算的多种羟酰胺配体与La 3+离子协同螯合的研究
为了研究与镧离子(La 3+)存在多个螯合配体羟酰胺(HA)分子以有效提取镧系元素。优化结构的几何分析,热化学分析,势能表面研究,电子密度分布确定和局部轨道定位器分析,分子轨道分析,状态密度分析和由一,二,三组成的复合物的振动光谱(IR光谱)分析,并且已经完成了具有镧的四个羟酰胺配体。计算出的几何参数,相互作用能,吉布自由能的变化,焓的变化,熵的变化以及HOMO-LUMO研究表明,随着更多数量的羟酰胺配体与La(III)。结构分析和振动分析表明羟酰胺和镧之间形成氢键状的非键相互作用。轨道分析提供了镧与羟基酰胺配体中碳的双键键合氧原子之间键形成的根本原因。上述研究有助于分析镧与羟酰胺的相互作用,以设计螯合萃取剂,以有效地萃取这种稀土元素。











































 京公网安备 11010802027423号
京公网安备 11010802027423号