Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Basis and Kinetic Pathway of RBM39 Recruitment to DCAF15 by a Sulfonamide Molecular Glue E7820.
Structure ( IF 4.4 ) Pub Date : 2019-11-05 , DOI: 10.1016/j.str.2019.10.005 Xinlin Du 1 , Oleg A Volkov 1 , Robert M Czerwinski 1 , HuiLing Tan 1 , Carlos Huerta 1 , Emily R Morton 1 , Jim P Rizzi 1 , Paul M Wehn 1 , Rui Xu 1 , Deepak Nijhawan 2 , Eli M Wallace 1
Structure ( IF 4.4 ) Pub Date : 2019-11-05 , DOI: 10.1016/j.str.2019.10.005 Xinlin Du 1 , Oleg A Volkov 1 , Robert M Czerwinski 1 , HuiLing Tan 1 , Carlos Huerta 1 , Emily R Morton 1 , Jim P Rizzi 1 , Paul M Wehn 1 , Rui Xu 1 , Deepak Nijhawan 2 , Eli M Wallace 1
Affiliation
|
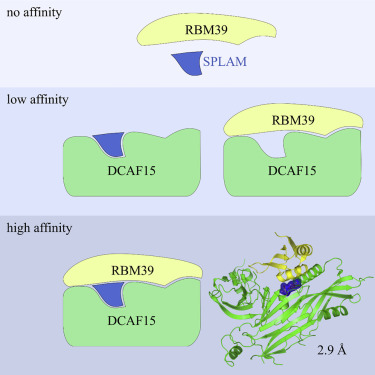
E7820 and indisulam are two examples of aryl sulfonamides that recruit RBM39 to Rbx-Cul4-DDA1-DDB1-DCAF15 E3 ligase complex, leading to its ubiquitination and degradation by the proteasome. To understand their mechanism of action, we performed kinetic analysis on the recruitment of RBM39 to DCAF15 and solved a crystal structure of DDA1-DDB1-DCAF15 in complex with E7820 and the RRM2 domain of RBM39. E7820 packs in a shallow pocket on the surface of DCAF15 and the resulting modified interface binds RBM39 through the α1 helix of the RRM2 domain. Our kinetic studies revealed that aryl sulfonamide and RBM39 bind to DCAF15 in a synergistic manner. The structural and kinetic studies confirm aryl sulfonamides as molecular glues in the recruitment of RBM39 and provide a framework for future efforts to utilize DCAF15 to degrade other proteins of interest.
中文翻译:

磺酰胺分子胶E7820的RBM39募集到DCAF15的结构基础和动力学途径。
E7820和靛蓝是芳基磺酰胺的两个实例,它们将RBM39募集到Rbx-Cul4-DDA1-DDB1-DCAF15 E3连接酶复合物,导致其泛素化并被蛋白酶体降解。为了了解其作用机理,我们对RBM39募集到DCAF15进行了动力学分析,并解析了与E7820和RBM39的RRM2结构域复合的DDA1-DDB1-DCAF15的晶体结构。E7820装在DCAF15表面的一个浅口袋中,所得修饰的界面通过RRM2结构域的α1螺旋与RBM39结合。我们的动力学研究表明,芳基磺酰胺和RBM39以协同方式与DCAF15结合。结构和动力学研究证实了芳基磺酰胺是RBM39募集过程中的分子胶,并为将来使用DCAF15降解其他目的蛋白提供了框架。
更新日期:2019-11-05
中文翻译:

磺酰胺分子胶E7820的RBM39募集到DCAF15的结构基础和动力学途径。
E7820和靛蓝是芳基磺酰胺的两个实例,它们将RBM39募集到Rbx-Cul4-DDA1-DDB1-DCAF15 E3连接酶复合物,导致其泛素化并被蛋白酶体降解。为了了解其作用机理,我们对RBM39募集到DCAF15进行了动力学分析,并解析了与E7820和RBM39的RRM2结构域复合的DDA1-DDB1-DCAF15的晶体结构。E7820装在DCAF15表面的一个浅口袋中,所得修饰的界面通过RRM2结构域的α1螺旋与RBM39结合。我们的动力学研究表明,芳基磺酰胺和RBM39以协同方式与DCAF15结合。结构和动力学研究证实了芳基磺酰胺是RBM39募集过程中的分子胶,并为将来使用DCAF15降解其他目的蛋白提供了框架。











































 京公网安备 11010802027423号
京公网安备 11010802027423号