当前位置:
X-MOL 学术
›
Nat. Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural complementarity facilitates E7820-mediated degradation of RBM39 by DCAF15.
Nature Chemical Biology ( IF 12.9 ) Pub Date : 2019-11-04 , DOI: 10.1038/s41589-019-0378-3 Tyler B Faust 1, 2 , Hojong Yoon 1, 2 , Radosław P Nowak 1, 2 , Katherine A Donovan 1, 2 , Zhengnian Li 1, 2 , Quan Cai 1, 2 , Nicholas A Eleuteri 1, 2 , Tinghu Zhang 1, 2 , Nathanael S Gray 1, 2 , Eric S Fischer 1, 2
Nature Chemical Biology ( IF 12.9 ) Pub Date : 2019-11-04 , DOI: 10.1038/s41589-019-0378-3 Tyler B Faust 1, 2 , Hojong Yoon 1, 2 , Radosław P Nowak 1, 2 , Katherine A Donovan 1, 2 , Zhengnian Li 1, 2 , Quan Cai 1, 2 , Nicholas A Eleuteri 1, 2 , Tinghu Zhang 1, 2 , Nathanael S Gray 1, 2 , Eric S Fischer 1, 2
Affiliation
|
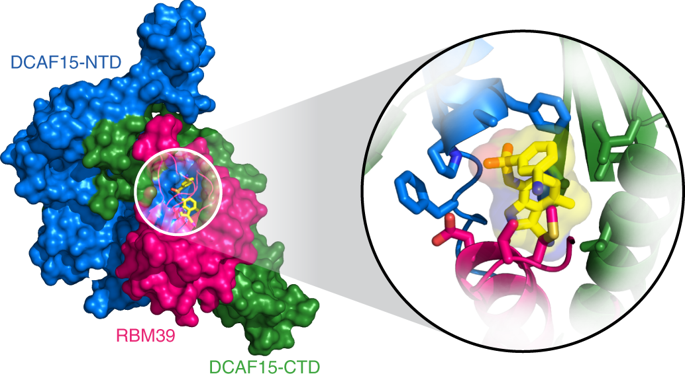
The investigational drugs E7820, indisulam and tasisulam (aryl-sulfonamides) promote the degradation of the splicing factor RBM39 in a proteasome-dependent mechanism. While the activity critically depends on the cullin RING ligase substrate receptor DCAF15, the molecular details remain elusive. Here we present the cryo-EM structure of the DDB1-DCAF15-DDA1 core ligase complex bound to RBM39 and E7820 at a resolution of 4.4 Å, together with crystal structures of engineered subcomplexes. We show that DCAF15 adopts a new fold stabilized by DDA1, and that extensive protein-protein contacts between the ligase and substrate mitigate low affinity interactions between aryl-sulfonamides and DCAF15. Our data demonstrate how aryl-sulfonamides neo-functionalize a shallow, non-conserved pocket on DCAF15 to selectively bind and degrade RBM39 and the closely related splicing factor RBM23 without the requirement for a high-affinity ligand, which has broad implications for the de novo discovery of molecular glue degraders.
中文翻译:

结构互补性促进 E7820 介导的 DCAF15 对 RBM39 的降解。
研究药物 E7820、indisulam 和 tasisulam(芳基磺胺类药物)以蛋白酶体依赖性机制促进剪接因子 RBM39 的降解。虽然活性严重依赖于 cullin RING 连接酶底物受体 DCAF15,但分子细节仍然难以捉摸。在这里,我们以 4.4 Å 的分辨率展示了与 RBM39 和 E7820 结合的 DDB1-DCAF15-DDA1 核心连接酶复合物的冷冻电镜结构,以及工程亚复合物的晶体结构。我们表明 DCAF15 采用了由 DDA1 稳定的新折叠,并且连接酶和底物之间广泛的蛋白质 - 蛋白质接触减轻了芳基磺酰胺和 DCAF15 之间的低亲和力相互作用。我们的数据证明了芳基磺胺类药物是如何对浅层进行新功能化的,
更新日期:2019-11-04
中文翻译:

结构互补性促进 E7820 介导的 DCAF15 对 RBM39 的降解。
研究药物 E7820、indisulam 和 tasisulam(芳基磺胺类药物)以蛋白酶体依赖性机制促进剪接因子 RBM39 的降解。虽然活性严重依赖于 cullin RING 连接酶底物受体 DCAF15,但分子细节仍然难以捉摸。在这里,我们以 4.4 Å 的分辨率展示了与 RBM39 和 E7820 结合的 DDB1-DCAF15-DDA1 核心连接酶复合物的冷冻电镜结构,以及工程亚复合物的晶体结构。我们表明 DCAF15 采用了由 DDA1 稳定的新折叠,并且连接酶和底物之间广泛的蛋白质 - 蛋白质接触减轻了芳基磺酰胺和 DCAF15 之间的低亲和力相互作用。我们的数据证明了芳基磺胺类药物是如何对浅层进行新功能化的,











































 京公网安备 11010802027423号
京公网安备 11010802027423号