当前位置:
X-MOL 学术
›
Beilstein. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
AgNTf2-catalyzed formal [3 + 2] cycloaddition of ynamides with unprotected isoxazol-5-amines: efficient access to functionalized 5-amino-1H-pyrrole-3-carboxamide derivatives
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2019-11-04 , DOI: 10.3762/bjoc.15.255 Ziping Cao , Jiekun Zhu , Li Liu , Yuanling Pang , Laijin Tian , Xuejun Sun , Xin Meng
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2019-11-04 , DOI: 10.3762/bjoc.15.255 Ziping Cao , Jiekun Zhu , Li Liu , Yuanling Pang , Laijin Tian , Xuejun Sun , Xin Meng
|
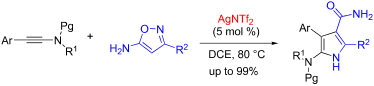
A formal [3 + 2] cycloaddition between ynamides and unprotected isoxazol-5-amines has been developed in the presence of catalytic AgNTf2 in an open flask. By the protocol, a variety of functionalized 5-amino-1H-pyrrole-3-carboxamide derivatives can be obtained in up to 99% yield. The reaction mechanism might involve the generation of an unusual α-imino silver carbene intermediate (or a silver-stabilized carbocation) and subsequent cyclization/isomerization to build the significant pyrrole-3-carboxamide motif. The reaction features the use of an inexpensive catalyst, simple reaction conditions, simple work-up without column chromatographic purification for most of products and high yields.
中文翻译:

AgNTf2催化的未保护的异恶唑-5-胺类酰胺的正式[3 + 2]环加成反应:有效获得官能化的5-氨基-1H-吡咯-3-羧酰胺衍生物
在开放式烧瓶中,在催化的AgNTf 2存在下,开发了酰胺和未保护的异恶唑-5-胺之间的正式[3 + 2]环加成反应。通过该协议,可以以高达99%的收率获得各种功能化的5-氨基-1 H-吡咯-3-羧酰胺衍生物。反应机理可能涉及生成异常的α-亚氨基亚银卡宾中间体(或银稳定的碳正离子)和随后的环化/异构化,以构建显着的吡咯-3-羧酰胺基序。该反应的特点是使用便宜的催化剂,简单的反应条件,无需柱色谱纯化即可进行简单后处理的大多数产品,并且收率很高。
更新日期:2019-11-04
中文翻译:

AgNTf2催化的未保护的异恶唑-5-胺类酰胺的正式[3 + 2]环加成反应:有效获得官能化的5-氨基-1H-吡咯-3-羧酰胺衍生物
在开放式烧瓶中,在催化的AgNTf 2存在下,开发了酰胺和未保护的异恶唑-5-胺之间的正式[3 + 2]环加成反应。通过该协议,可以以高达99%的收率获得各种功能化的5-氨基-1 H-吡咯-3-羧酰胺衍生物。反应机理可能涉及生成异常的α-亚氨基亚银卡宾中间体(或银稳定的碳正离子)和随后的环化/异构化,以构建显着的吡咯-3-羧酰胺基序。该反应的特点是使用便宜的催化剂,简单的反应条件,无需柱色谱纯化即可进行简单后处理的大多数产品,并且收率很高。











































 京公网安备 11010802027423号
京公网安备 11010802027423号