当前位置:
X-MOL 学术
›
Beilstein. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Arylisoquinoline-derived organoboron dyes with a triaryl skeleton show dual fluorescence
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2019-11-04 , DOI: 10.3762/bjoc.15.254 Vânia F Pais 1 , Tristan Neumann 1 , Ignacio Vayá 2 , M Consuelo Jiménez 2 , Abel Ros 3, 4 , Uwe Pischel 1
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2019-11-04 , DOI: 10.3762/bjoc.15.254 Vânia F Pais 1 , Tristan Neumann 1 , Ignacio Vayá 2 , M Consuelo Jiménez 2 , Abel Ros 3, 4 , Uwe Pischel 1
Affiliation
|
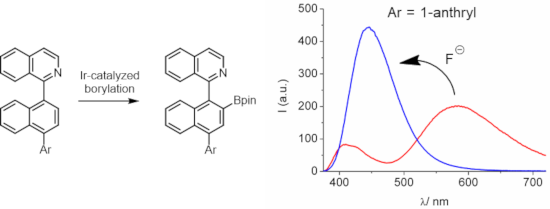
Four new dyes that derive from borylated arylisoquinolines were prepared, containing a third aryl residue (naphthyl, 4-methoxynaphthyl, pyrenyl or anthryl) that is linked via an additional stereogenic axis. The triaryl cores were synthesized by Suzuki couplings and then transformed into boronic acid esters by employing an Ir(I)-catalyzed reaction. The chromophores show dual emission behavior, where the long-wavelength emission band can reach maxima close to 600 nm in polar solvents. The fluorescence quantum yields of the dyes are generally in the range of 0.2–0.4, reaching in some cases values as high as 0.5–0.6. Laser-flash photolysis provided evidence for the existence of excited triplet states. The dyes form fluoroboronate complexes with fluoride anions, leading to the observation of the quenching of the long-wavelength emission band and ratiometric response by the build-up of a hypsochromically shifted emission signal.
中文翻译:

具有三芳基骨架的芳基异喹啉衍生的有机硼染料显示出双荧光
制备了四种源自硼基化芳基异喹啉的新染料,其中含有通过额外立体轴连接的第三个芳基残基(萘基、4-甲氧基萘基、芘基或蒽基)。三芳基核通过 Suzuki 偶联合成,然后通过 Ir(I) 催化反应转化为硼酸酯。发色团表现出双发射行为,其中长波长发射带在极性溶剂中可以达到接近 600 nm 的最大值。染料的荧光量子产率通常在0.2-0.4的范围内,在某些情况下达到高达0.5-0.6的值。激光闪光光解提供了激发三重态存在的证据。这些染料与氟阴离子形成氟硼酸盐络合物,从而观察到长波长发射带的猝灭和通过建立低色位移发射信号而产生的比例响应。
更新日期:2019-11-04
中文翻译:

具有三芳基骨架的芳基异喹啉衍生的有机硼染料显示出双荧光
制备了四种源自硼基化芳基异喹啉的新染料,其中含有通过额外立体轴连接的第三个芳基残基(萘基、4-甲氧基萘基、芘基或蒽基)。三芳基核通过 Suzuki 偶联合成,然后通过 Ir(I) 催化反应转化为硼酸酯。发色团表现出双发射行为,其中长波长发射带在极性溶剂中可以达到接近 600 nm 的最大值。染料的荧光量子产率通常在0.2-0.4的范围内,在某些情况下达到高达0.5-0.6的值。激光闪光光解提供了激发三重态存在的证据。这些染料与氟阴离子形成氟硼酸盐络合物,从而观察到长波长发射带的猝灭和通过建立低色位移发射信号而产生的比例响应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号