Insect Biochemistry and Molecular Biology ( IF 3.2 ) Pub Date : 2019-11-04 , DOI: 10.1016/j.ibmb.2019.103261 Yang Wang 1 , Fan Yang 1 , Xiaolong Cao 1 , Rudan Huang 1 , Susan Paskewitz 2 , Steve D Hartson 3 , Michael R Kanost 4 , Haobo Jiang 1
|
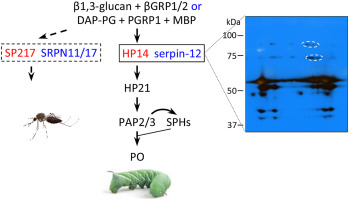
A network of serine proteases (SPs) and their non-catalytic homologs (SPHs) activates prophenoloxidase (proPO), Toll pathway, and other insect immune responses. However, integration and conservation of the network and its control mechanisms have not yet been fully understood. Here we present evidence that these responses are initiated through a conserved serine protease and negatively regulated by serpins in two species, Manduca sexta and Anopheles gambiae. We have shown that M. sexta serpin-12 reduces the proteolytic activation of HP6, HP8, proPO activating proteases (PAPs), SPHs, and POs in larval hemolymph, and we hypothesized that these effects are due to the inhibition of the immune pathway-initiating protease HP14. To test whether these changes are due to HP14 inhibition, we isolated a covalent complex of HP14 with serpin-12 from plasma using polyclonal antibodies against the HP14 protease domain or against serpin-12, and confirmed formation of the complex by 2D-electrophoresis, immunoblotting, and mass spectrometry. Upon recognition of bacterial peptidoglycans or fungal β-1,3-glucan, the zymogen proHP14 became active HP14, which formed an SDS-stable complex with serpin-12 in vitro. Activation of proHP21 by HP14 was suppressed by serpin-12, consistent with the decrease in steps downstream of HP21, proteolytic activation of proPAP3, proSPH1/2 and proPO in hemolymph. Guided by the results of phylogenetic analysis, we cloned and expressed A. gambiae proSP217 (an ortholog of HP14) and core domains of A. gambiae serpin-11 and -17. The recombinant SP217 zymogen became active during expression, with cleavage between Tyr394 and Ile395. Both MsHP14 and AgSP217 cleaved MsSerpin-12 and AgSRPN11 at Leu*Ser (P1*P1’) and formed complexes in vitro. ProPO activation in M. sexta plasma increased after recombinant AgSP217 had been added, indicating that it may function in a similar manner as the endogenous initiating protease HP14. Based on these data, we propose that inhibition of an initiating modular protease by a serpin may be a common mechanism in holometabolous insects to regulate proPO activation and other protease-induced immune responses.
中文翻译:

Manduca sexta serpin-12 对免疫途径启动血淋巴蛋白酶 14 的抑制,这是调节昆虫黑化和 Toll 激活的保守机制
丝氨酸蛋白酶 (SP) 及其非催化同系物 (SPH) 网络可激活酚氧化酶原 (proPO)、Toll 途径和其他昆虫免疫反应。然而,网络的整合和保护及其控制机制尚未得到充分理解。在这里,我们提供的证据表明,这些反应是通过保守的丝氨酸蛋白酶启动的,并受到烟草天蛾和冈比亚按蚊这两个物种中丝氨酸蛋白酶抑制剂的负调控。我们已经证明, M. sexta serpin-12 降低了幼虫血淋巴中 HP6、HP8、proPO 激活蛋白酶 (PAP)、SPH 和 PO 的蛋白水解活性,我们假设这些作用是由于免疫途径的抑制所致 -启动蛋白酶HP14。为了测试这些变化是否是由于 HP14 抑制所致,我们使用针对 HP14 蛋白酶结构域或针对 serpin-12 的多克隆抗体从血浆中分离出 HP14 与 serpin-12 的共价复合物,并通过 2D 电泳、免疫印迹证实了复合物的形成和质谱分析。在识别细菌肽聚糖或真菌β-1,3-葡聚糖后,酶原proHP14变成活性HP14,在体外与serpin-12形成SDS稳定的复合物。 HP14 对 proHP21 的激活被 serpin-12 抑制,这与 HP21 下游步骤的减少、血淋巴中 proPAP3、proSPH1/2 和 proPO 的蛋白水解激活一致。在系统发育分析结果的指导下,我们克隆并表达了冈比亚曲霉proSP217(HP14的直系同源物)以及冈比亚曲霉serpin-11和-17的核心结构域。重组 SP217 酶原在表达过程中变得活跃,在 Tyr 394和 Ile 395之间裂解。 MsHP14 和 AgSP217 均在 Leu*Ser (P1*P1') 处裂解 MsSerpin-12 和 AgSRPN11,并在体外形成复合物。添加重组AgSP217后, M. sexta血浆中的ProPO活化增加,表明其可能以与内源起始蛋白酶HP14类似的方式发挥作用。基于这些数据,我们提出,丝氨酸蛋白酶抑制剂对起始模块化蛋白酶的抑制可能是全变态昆虫调节 proPO 激活和其他蛋白酶诱导的免疫反应的常见机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号