当前位置:
X-MOL 学术
›
Magn. Reson. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Purity determination of a new antifungal drug candidate using quantitative 1 H NMR spectroscopy: method validation and comparison of calibration approaches
Magnetic Resonance in Chemistry ( IF 1.9 ) Pub Date : 2019-09-01 , DOI: 10.1002/mrc.4936 Pedro Henrique Cavalcanti Franco 1 , Saulo Fehelberg Pinto Braga 2 , Renata Barbosa de Oliveira 1 , Isabela Costa César 1
Magnetic Resonance in Chemistry ( IF 1.9 ) Pub Date : 2019-09-01 , DOI: 10.1002/mrc.4936 Pedro Henrique Cavalcanti Franco 1 , Saulo Fehelberg Pinto Braga 2 , Renata Barbosa de Oliveira 1 , Isabela Costa César 1
Affiliation
|
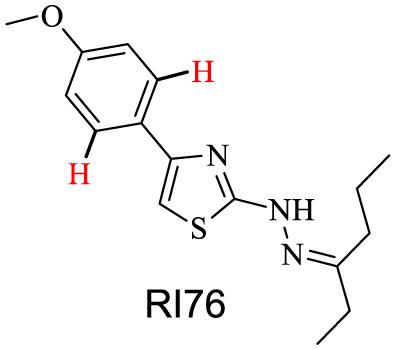
Quantitative nuclear magnetic resonance (qNMR) is an analytical technique that offers numerous advantages in pharmaceutical applications including minimum sample preparation and rapid data collection times with no need for response factor corrections, being a powerful tool for assaying drug content in both drug discovery and early drug development. In the present work, we have applied qNMR, using both the internal standard and the electronic reference to access in vivo concentrations 2 calibration methods, to assess the purity of RI76, a novel antifungal drug candidate. NMR acquisition and processing parameters were optimized in order to obtain spectra with intense, well‐resolved signals of completely relaxed nuclei. The analytical method was validated following current guidelines, demonstrating selectivity, linearity, accuracy, precision, and robustness. The calibration approaches were statistically compared, and no significant difference was observed when comparing the obtained results and their dispersion in terms of relative standard deviation. The proposed qNMR method may, therefore, be used for both qualitative and quantitative assessments of RI76 in early drug development and for characterization of this compound.
中文翻译:

使用定量 1 H NMR 光谱测定新的抗真菌药物候选物的纯度:方法验证和校准方法的比较
定量核磁共振 (qNMR) 是一种分析技术,在制药应用中具有许多优势,包括最少的样品制备和快速的数据收集时间,无需响应因子校正,是在药物发现和早期药物分析中分析药物含量的有力工具发展。在目前的工作中,我们已应用 qNMR,同时使用内标和电子参考来访问体内浓度 2 校准方法,以评估 RI76(一种新型抗真菌药物候选物)的纯度。优化了 NMR 采集和处理参数,以获得具有完全松弛核的强烈、解析良好的信号的光谱。该分析方法按照当前指南进行了验证,证明了选择性、线性、准确度、精密度、和鲁棒性。对校准方法进行了统计比较,比较所得结果及其相对标准偏差的离散度时,没有观察到显着差异。因此,提议的 qNMR 方法可用于早期药物开发中 RI76 的定性和定量评估以及该化合物的表征。
更新日期:2019-09-01
中文翻译:

使用定量 1 H NMR 光谱测定新的抗真菌药物候选物的纯度:方法验证和校准方法的比较
定量核磁共振 (qNMR) 是一种分析技术,在制药应用中具有许多优势,包括最少的样品制备和快速的数据收集时间,无需响应因子校正,是在药物发现和早期药物分析中分析药物含量的有力工具发展。在目前的工作中,我们已应用 qNMR,同时使用内标和电子参考来访问体内浓度 2 校准方法,以评估 RI76(一种新型抗真菌药物候选物)的纯度。优化了 NMR 采集和处理参数,以获得具有完全松弛核的强烈、解析良好的信号的光谱。该分析方法按照当前指南进行了验证,证明了选择性、线性、准确度、精密度、和鲁棒性。对校准方法进行了统计比较,比较所得结果及其相对标准偏差的离散度时,没有观察到显着差异。因此,提议的 qNMR 方法可用于早期药物开发中 RI76 的定性和定量评估以及该化合物的表征。











































 京公网安备 11010802027423号
京公网安备 11010802027423号