当前位置:
X-MOL 学术
›
J. Mol. Recognit.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Study of tetraphenylporphyrins as modifiers of insulin amyloid aggregation.
Journal of Molecular Recognition ( IF 2.3 ) Pub Date : 2019-09-09 , DOI: 10.1002/jmr.2811 Svitlana Chernii 1 , Mykhaylo Losytskyy 1 , Anna Kelm 2 , Alexandr Gorski 2 , Iryna Tretyakova 3 , Sergiy Yarmoluk 1 , Victor Chernii 3 , Vladyslava Kovalska 1
Journal of Molecular Recognition ( IF 2.3 ) Pub Date : 2019-09-09 , DOI: 10.1002/jmr.2811 Svitlana Chernii 1 , Mykhaylo Losytskyy 1 , Anna Kelm 2 , Alexandr Gorski 2 , Iryna Tretyakova 3 , Sergiy Yarmoluk 1 , Victor Chernii 3 , Vladyslava Kovalska 1
Affiliation
|
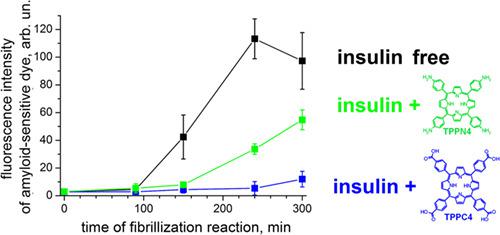
Amyloid fibrils are rigid β-pleated protein aggregates that are connected with series of harmful diseases and at the same time are promising as base for novel nanomaterials. Thus, design of compounds able to inhibit or redirect those aggregates formation is important both for the biomedical aims and for nanotechnology applications. Here, we studied the effect of tetraphenylporphyrins (metal free, their Cu and Pd complexes, and those functionalized by carboxy and amino groups on periphery) on insulin amyloid self-assembling. The strongest impact on insulin aggregation was demonstrated by a metal-free porphyrin bearing four carboxy groups. This compound strongly suppresses insulin aggregation (about 88% reduction in amyloid-sensitive probe emission) inducing formation of fibrils with the length close to this of free insulin (1.7 ± 0.6 μm as compared with 1.4 ± 0.4 μm, respectively) with an essentially reduced tendency to lateral aggregation. Contrarily, the presence of tetraphenylporphyrin containing four amino groups only slightly affects fibrils' morphology and makes weaker impact on insulin aggregation yield (about 44% reduction). This is explained by the ability of aromatic carboxy groups of 5,10,15,20-(tetra-4-carboxyphenyl)porphyrin to interact with complementary protein-binding groups and thus stabilize the supramolecular complex. For 5,10,15,20-(tetra-4-aminophenyl)porphyrin, full protonation takes place in acidic medium of protein aggregation reaction; this results in the high positive charge of TPPN4 (equal or close to +6) and hence higher contribution of coulombic repulsion to interaction of TPPN4 with insulin. One more possible mechanism of the lower inhibition effect of TPPN4 as compared with TPPC4 could be the more restricted possibility of the former as compared with the latter to form H bonds with insulin groups. It was also shown that metal-free, Pd-containing, and Cu-containing tetraphenylporphyrins without peripheral substituents make almost the same impact on the protein self-assembling. We suppose this to be due to coordination saturation of these metal atoms.
中文翻译:

四苯基卟啉作为胰岛素淀粉样蛋白聚集修饰剂的研究。
淀粉样原纤维是刚性的β-折叠蛋白聚集体,其与一系列有害疾病有关,同时有望作为新型纳米材料的基础。因此,设计能够抑制或重定向那些聚集体形成的化合物对于生物医学目的和纳米技术应用都是重要的。在这里,我们研究了四苯基卟啉(无金属,它们的Cu和Pd络合物以及通过外围的羧基和氨基官能化的那些)对胰岛素淀粉样蛋白自组装的影响。带有四个羧基的无金属卟啉证明了对胰岛素聚集的最强影响。该化合物强烈抑制胰岛素聚集(淀粉样敏感性探针发射减少约88%),诱导原纤维形成,其长度接近游离胰岛素的原纤维长度(1.7±0。与分别为1.4±0.4μm的6μm相比,横向聚集的趋势大大降低。相反,含有四个氨基的四苯基卟啉的存在仅轻微影响原纤维的形态,并且对胰岛素聚集产率的影响较弱(降低约44%)。这由5,10,15,20-(四-4-羧基苯基)卟啉的芳香族羧基与互补的蛋白质结合基团相互作用从而稳定超分子复合物的能力解释。对于5,10,15,20-(四-4-氨基苯基)卟啉,在蛋白质聚集反应的酸性介质中发生完全质子化。这导致TPPN4的正电荷较高(等于或接近+6),因此库仑排斥对TPPN4与胰岛素相互作用的贡献更大。与TPPC4相比,TPPN4抑制作用更低的另一种可能的机制可能是前者与后者相比与胰岛素基团形成H键的可能性受到更大的限制。还显示了无金属,无钯和含铜的四苯基卟啉,而没有外围取代基对蛋白质的自组装产生几乎相同的影响。我们认为这是由于这些金属原子的配位饱和。
更新日期:2019-12-13
中文翻译:

四苯基卟啉作为胰岛素淀粉样蛋白聚集修饰剂的研究。
淀粉样原纤维是刚性的β-折叠蛋白聚集体,其与一系列有害疾病有关,同时有望作为新型纳米材料的基础。因此,设计能够抑制或重定向那些聚集体形成的化合物对于生物医学目的和纳米技术应用都是重要的。在这里,我们研究了四苯基卟啉(无金属,它们的Cu和Pd络合物以及通过外围的羧基和氨基官能化的那些)对胰岛素淀粉样蛋白自组装的影响。带有四个羧基的无金属卟啉证明了对胰岛素聚集的最强影响。该化合物强烈抑制胰岛素聚集(淀粉样敏感性探针发射减少约88%),诱导原纤维形成,其长度接近游离胰岛素的原纤维长度(1.7±0。与分别为1.4±0.4μm的6μm相比,横向聚集的趋势大大降低。相反,含有四个氨基的四苯基卟啉的存在仅轻微影响原纤维的形态,并且对胰岛素聚集产率的影响较弱(降低约44%)。这由5,10,15,20-(四-4-羧基苯基)卟啉的芳香族羧基与互补的蛋白质结合基团相互作用从而稳定超分子复合物的能力解释。对于5,10,15,20-(四-4-氨基苯基)卟啉,在蛋白质聚集反应的酸性介质中发生完全质子化。这导致TPPN4的正电荷较高(等于或接近+6),因此库仑排斥对TPPN4与胰岛素相互作用的贡献更大。与TPPC4相比,TPPN4抑制作用更低的另一种可能的机制可能是前者与后者相比与胰岛素基团形成H键的可能性受到更大的限制。还显示了无金属,无钯和含铜的四苯基卟啉,而没有外围取代基对蛋白质的自组装产生几乎相同的影响。我们认为这是由于这些金属原子的配位饱和。











































 京公网安备 11010802027423号
京公网安备 11010802027423号