当前位置:
X-MOL 学术
›
J. Chin. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism of synthesis of anatase TiO2 pigment from low concentration of titanyl sulfuric–chloric acid solution under hydrothermal hydrolysis
Journal of the Chinese Chemical Society ( IF 1.6 ) Pub Date : 2019-07-17 , DOI: 10.1002/jccs.201900071 Ming Tian 1, 2 , Yahui Liu 1, 2 , Weijing Wang 1, 2 , Wei Zhao 1, 2 , Desheng Chen 1, 2 , Lina Wang 1, 2 , Hongxin Zhao 1, 2 , Fancheng Meng 1, 2 , Yulan Zhen 1, 2 , Zongyuan Hu 2 , Tao Qi 1, 2
Journal of the Chinese Chemical Society ( IF 1.6 ) Pub Date : 2019-07-17 , DOI: 10.1002/jccs.201900071 Ming Tian 1, 2 , Yahui Liu 1, 2 , Weijing Wang 1, 2 , Wei Zhao 1, 2 , Desheng Chen 1, 2 , Lina Wang 1, 2 , Hongxin Zhao 1, 2 , Fancheng Meng 1, 2 , Yulan Zhen 1, 2 , Zongyuan Hu 2 , Tao Qi 1, 2
Affiliation
|
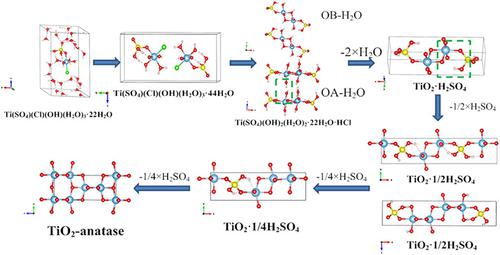
In order to investigate the influence of Cl−/SO42− molar ratios and hydrolysis temperature on the hydrolysis process and TiO2 pigment, H2TiO3 was prepared with a low concentration of titanyl sulfuric–chloric acid solution by hydrothermal hydrolysis. Under the optimal hydrolysis conditions, 1.5–2.2 μm of H2TiO3 samples were achieved. After doping and calcination, anatase TiO2 pigments demonstrated excellent performance: the achromic ability of tinctorial strength (TCS) and blue phase index (SCX) were 1,429 and 4.07, respectively. As hydrolysis was a significant step in the process, the structure was simplified to a periodic structure of Ti[OH](H2O)3Cl(SO4) to simulate the cluster structures. Based on experimental results and density functional theory (DFT) calculation, the hydrolysis mechanism was presumed to be a process of anionic (OH−, Cl− and SO42−) competition reaction to explain the formation of anatase‐type H2TiO3, and the crystal growth direction of H2TiO3 was also confirmed to be a (OA) and b (OB).
中文翻译:

水热水解低浓度钛酰硫酸-盐酸溶液合成锐钛矿型TiO2颜料的机理。
为了调查Cl组成的影响- / SO 4 2-的水解过程和TiO摩尔比和水解温度2颜料,H 2的TiO 3用由水热水解的低浓度的氧钛硫酸-氯酸溶液制备。在最佳水解条件下,获得了1.5–2.2μm的H 2 TiO 3样品。掺杂和煅烧后,锐钛矿型TiO 2颜料表现出优异的性能:着色力(TCS)和蓝相指数(SCX)的消色力分别为1,429和4.07。由于水解是该过程中的重要步骤,因此将结构简化为Ti [OH](H 2 O)3 Cl(SO 4)的周期性结构,以模拟团簇结构。基于实验结果和密度泛函理论(DFT)计算,水解机制推测是阴离子的处理(OH - ,氯-和SO 4 2-)反应的竞争来解释锐钛矿型H的形成2的TiO 3,以及H 2 TiO的晶体生长方向3也被确认为a(OA)和b(OB)。
更新日期:2020-02-14
中文翻译:

水热水解低浓度钛酰硫酸-盐酸溶液合成锐钛矿型TiO2颜料的机理。
为了调查Cl组成的影响- / SO 4 2-的水解过程和TiO摩尔比和水解温度2颜料,H 2的TiO 3用由水热水解的低浓度的氧钛硫酸-氯酸溶液制备。在最佳水解条件下,获得了1.5–2.2μm的H 2 TiO 3样品。掺杂和煅烧后,锐钛矿型TiO 2颜料表现出优异的性能:着色力(TCS)和蓝相指数(SCX)的消色力分别为1,429和4.07。由于水解是该过程中的重要步骤,因此将结构简化为Ti [OH](H 2 O)3 Cl(SO 4)的周期性结构,以模拟团簇结构。基于实验结果和密度泛函理论(DFT)计算,水解机制推测是阴离子的处理(OH - ,氯-和SO 4 2-)反应的竞争来解释锐钛矿型H的形成2的TiO 3,以及H 2 TiO的晶体生长方向3也被确认为a(OA)和b(OB)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号