当前位置:
X-MOL 学术
›
J. Chin. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Study of intramolecular interactions in aspirin
Journal of the Chinese Chemical Society ( IF 1.6 ) Pub Date : 2019-05-20 , DOI: 10.1002/jccs.201900055 Sandra Cotes 1 , Jose Cotuá 2
Journal of the Chinese Chemical Society ( IF 1.6 ) Pub Date : 2019-05-20 , DOI: 10.1002/jccs.201900055 Sandra Cotes 1 , Jose Cotuá 2
Affiliation
|
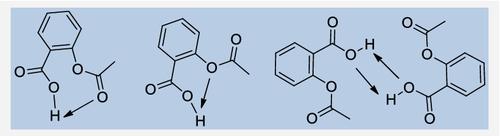
A detailed quantum chemical study of the solvent effects in the intramolecular hydrogen bonding, conformational stability, and reactivity of aspirin has been performed using density functional theory (DFT) at the B3LYP/6‐31G(d) theory level. Seven conformational isomers, three of them presenting intramolecular hydrogen bonds, have been located. Thermochemical functions have been computed, and relative energies and free enthalpies have been determined in gas and aqueous phases. Several molecular properties have been calculated to predict the ability of aspirin to acylate cyclooxygenase (COX) enzymes. A six‐membered‐ring hydrogen‐bonded conformer was found to be the most reactive species. The solvation in aqueous phase increases the reactivity and strengthens intramolecular hydrogen bonding.
中文翻译:

阿司匹林分子内相互作用的研究
已在B3LYP / 6-31G(d)理论水平上使用密度泛函理论(DFT)对分子内氢键,构象稳定性和阿司匹林的溶剂作用进行了详细的量子化学研究。已经找到了七个构象异构体,其中三个呈现分子内氢键。已经计算出热化学函数,并且已经确定了气相和水相中的相对能量和自由焓。已经计算出几种分子特性来预测阿司匹林酰化环氧合酶(COX)酶的能力。发现六元环氢键键合构象异构体是最活泼的物种。在水相中的溶剂化增加了反应性并增强了分子内氢键。
更新日期:2019-12-11
中文翻译:

阿司匹林分子内相互作用的研究
已在B3LYP / 6-31G(d)理论水平上使用密度泛函理论(DFT)对分子内氢键,构象稳定性和阿司匹林的溶剂作用进行了详细的量子化学研究。已经找到了七个构象异构体,其中三个呈现分子内氢键。已经计算出热化学函数,并且已经确定了气相和水相中的相对能量和自由焓。已经计算出几种分子特性来预测阿司匹林酰化环氧合酶(COX)酶的能力。发现六元环氢键键合构象异构体是最活泼的物种。在水相中的溶剂化增加了反应性并增强了分子内氢键。











































 京公网安备 11010802027423号
京公网安备 11010802027423号