当前位置:
X-MOL 学术
›
Toxicology
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Amelioration of ochratoxin-A induced cytotoxicity by prophylactic treatment of N-Acetyl-L-Tryptophan in human embryonic kidney cells.
Toxicology ( IF 4.8 ) Pub Date : 2019-11-01 , DOI: 10.1016/j.tox.2019.152324 Prerna Agarwal 1 , Darshana Singh 1 , Sheikh Raisuddin 2 , Raj Kumar 1
Toxicology ( IF 4.8 ) Pub Date : 2019-11-01 , DOI: 10.1016/j.tox.2019.152324 Prerna Agarwal 1 , Darshana Singh 1 , Sheikh Raisuddin 2 , Raj Kumar 1
Affiliation
|
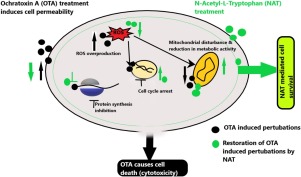
Ochratoxin A (OTA) is known to induce nephro-toxicity via induction of cellular redox homeostasis perturbation, mitochondrial hyperpolarisation and depolarization, protein synthesis inhibition leading to apoptosis. In the present study, protective efficacy of N-Acetyl-L-Tryptophan (NAT) against OTA induced toxicity was evaluated using Human Embryonic Kidney (HEK-293) cells. Cells were treated with NAT (0-200 μg/ml) before OTA treatment (0-20 μg/ml) and protective efficacy of NAT was evaluated using MTT and SRB assay. OTA-induced intracellular ROS generation and its inhibition by NAT (2.5 μg/ml) pre-treatment was evaluated using 2',7'-dichlorodihydrofluorescein diacetate (H2DCFDA) probe. Effects of NAT pre-treatment on OTA treated cells were also evaluated in terms of cell cycle perturbations and mitochondrial membrane potential disturbance using flowcytometry. Results of the study demonstrated significant (∼89 % cell growth in comparison to 50% in OTA alone group; P < 0.05) protection by NAT to the HEK-293 cells against OTA mediated cell death in terms of cell viability. Further, significant reduction in ROS levels and mitochondrial membrane potential disturbance was also observed in NAT pre-treated plus OTA cells as compared to only OTA treated cells. Significant (p < 0.05) arrest in G0 and S phase of cell cycle was observed in OTA treated cells that was found to be inhibited by NAT pre-treatment to OTA treated cells. Also, molecular docking analysis demonstrated higher probability of NAT to bind with OTA binding pocket on phenylalanyl t-RNA synthetase, resulting in inhibition of OTA incorporation in the newly synthesized peptides and thus may ameliorate OTA induced protein synthesis inhibition. Conclusively, present study suggested that NAT offers protection against OTA toxicity in HEK-293 cells by counterbalancing oxidative stress, cell cycle regulation, mitochondrial membrane potential stabilization, protein synthesis inhibition and cell death retardation.
中文翻译:

通过预防性处理人胚肾细胞中的N-乙酰基-L-色氨酸改善曲霉毒素A诱导的细胞毒性。
ch曲毒素A(OTA)已知通过诱导细胞氧化还原稳态扰动,线粒体超极化和去极化,蛋白合成抑制导致细胞凋亡来诱导肾毒性。在本研究中,使用人胚肾(HEK-293)细胞评估了N-乙酰基-L-色氨酸(NAT)对OTA诱导的毒性的保护作用。在OTA处理(0-20μg/ ml)之前,先用NAT(0-200μg/ ml)处理细胞,并使用MTT和SRB分析评估NAT的保护功效。使用2',7'-dichlorodihydrofluorescein diacetate(H2DCFDA)探针评估了OTA诱导的细胞内ROS的产生及其被NAT(2.5μg/ ml)预处理的抑制作用。还使用流式细胞术根据细胞周期扰动和线粒体膜电位障碍评估了NAT预处理对OTA处理的细胞的影响。研究结果表明,就细胞活力而言,NAT对HEK-293细胞具有显着的保护作用(〜89%的细胞生长,而单独使用OTA的占50%; P <0.05),以防止OTA介导的细胞死亡。此外,与仅OTA处理的细胞相比,在NAT预处理的加上OTA细胞中也观察到ROS水平和线粒体膜电位干扰的显着降低。在OTA处理的细胞中观察到细胞周期的G0和S期显着(p <0.05)停滞,这被NAT预处理对OTA处理的细胞所抑制。还,分子对接分析表明,NAT与苯丙氨酰t-RNA合成酶上OTA结合口袋结合的可能性更高,从而导致OTA掺入新合成的肽中而受到抑制,因此可以减轻OTA诱导的蛋白质合成抑制。总而言之,目前的研究表明,NAT可通过平衡氧化应激,细胞周期调节,线粒体膜电位稳定,蛋白质合成抑制和细胞死亡延迟来保护HEK-293细胞免受OTA毒性。
更新日期:2019-11-01
中文翻译:

通过预防性处理人胚肾细胞中的N-乙酰基-L-色氨酸改善曲霉毒素A诱导的细胞毒性。
ch曲毒素A(OTA)已知通过诱导细胞氧化还原稳态扰动,线粒体超极化和去极化,蛋白合成抑制导致细胞凋亡来诱导肾毒性。在本研究中,使用人胚肾(HEK-293)细胞评估了N-乙酰基-L-色氨酸(NAT)对OTA诱导的毒性的保护作用。在OTA处理(0-20μg/ ml)之前,先用NAT(0-200μg/ ml)处理细胞,并使用MTT和SRB分析评估NAT的保护功效。使用2',7'-dichlorodihydrofluorescein diacetate(H2DCFDA)探针评估了OTA诱导的细胞内ROS的产生及其被NAT(2.5μg/ ml)预处理的抑制作用。还使用流式细胞术根据细胞周期扰动和线粒体膜电位障碍评估了NAT预处理对OTA处理的细胞的影响。研究结果表明,就细胞活力而言,NAT对HEK-293细胞具有显着的保护作用(〜89%的细胞生长,而单独使用OTA的占50%; P <0.05),以防止OTA介导的细胞死亡。此外,与仅OTA处理的细胞相比,在NAT预处理的加上OTA细胞中也观察到ROS水平和线粒体膜电位干扰的显着降低。在OTA处理的细胞中观察到细胞周期的G0和S期显着(p <0.05)停滞,这被NAT预处理对OTA处理的细胞所抑制。还,分子对接分析表明,NAT与苯丙氨酰t-RNA合成酶上OTA结合口袋结合的可能性更高,从而导致OTA掺入新合成的肽中而受到抑制,因此可以减轻OTA诱导的蛋白质合成抑制。总而言之,目前的研究表明,NAT可通过平衡氧化应激,细胞周期调节,线粒体膜电位稳定,蛋白质合成抑制和细胞死亡延迟来保护HEK-293细胞免受OTA毒性。









































 京公网安备 11010802027423号
京公网安备 11010802027423号