当前位置:
X-MOL 学术
›
Neurochem. Int.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rapid and persistent loss of TXNIP in HT22 neuronal cells under carbonyl and hyperosmotic stress.
Neurochemistry international ( IF 4.4 ) Pub Date : 2019-11-01 , DOI: 10.1016/j.neuint.2019.104585 Alcir Luiz Dafre 1 , Ariana Ern Schmitz 1 , Pamela Maher 2
Neurochemistry international ( IF 4.4 ) Pub Date : 2019-11-01 , DOI: 10.1016/j.neuint.2019.104585 Alcir Luiz Dafre 1 , Ariana Ern Schmitz 1 , Pamela Maher 2
Affiliation
|
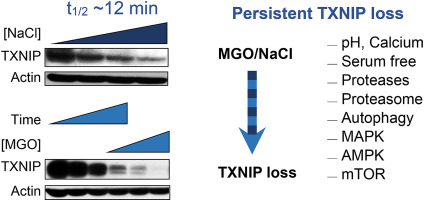
Thioredoxin interacting protein (TXNIP) binds to thioredoxin thereby limiting its activity, but it also promotes internalization of glucose transporters, participates in inflammasome activation, and controls autophagy. Published data and this work demonstrate that TXNIP responds to a number of apparently unrelated stresses, such as serum deprivation, pH change, and oxidative, osmotic and carbonyl stress. Interestingly, we noticed that hyperosmotic (NaCl) and carbonyl (methylglyoxal, MGO) stresses in HT22 neuronal cells produced a rapid loss of TXNIP (half-life ∼12 min), prompting us to search for possible mechanisms controlling this TXNIP loss, including pH change, serum deprivation, calcium metabolism and inhibition of the proteasome and other proteases, autophagy and MAPKs. None of these routes stopped the TXNIP loss induced by hyperosmotic and carbonyl stress. Besides transcriptional, translational and microRNA regulation, there is evidence indicating that mTOR and AMPK also control TXNIP expression. Indeed, AMPK-deficient mouse embryonic fibroblasts failed to respond to phenformin (AMPK activator) and compound C (AMPK inhibitor), while rapamycin induced a marked increase in TXNIP levels, confirming the known AMPK/mTOR control over TXNIP. However, the TXNIP loss induced by NaCl or MGO were observed even in AMPK deficient MEFs or after mTOR inhibition, indicating AMPK/mTOR does not participate in this rapid TXNIP loss. These results suggest that rapid TXNIP loss is a general and immediate response to stress that can improve energy availability and antioxidant protection, eventually culminating in better cell survival.
中文翻译:

在羰基和高渗应激下,HT22 神经元细胞中 TXNIP 的快速和持续丢失。
硫氧还蛋白相互作用蛋白 (TXNIP) 与硫氧还蛋白结合从而限制其活性,但它也促进葡萄糖转运蛋白的内化,参与炎性体激活并控制自噬。已发表的数据和这项工作表明 TXNIP 对许多明显无关的压力有反应,例如血清剥夺、pH 变化以及氧化、渗透压和羰基压力。有趣的是,我们注意到 HT22 神经元细胞中的高渗(NaCl)和羰基(甲基乙二醛,MGO)应激导致 TXNIP 快速损失(半衰期~12 分钟),促使我们寻找控制这种 TXNIP 损失的可能机制,包括 pH变化、血清剥夺、钙代谢和蛋白酶体和其他蛋白酶的抑制、自噬和 MAPK。这些途径都不能阻止由高渗和羰基胁迫引起的 TXNIP 损失。除了转录、翻译和 microRNA 调节外,有证据表明 mTOR 和 AMPK 也控制 TXNIP 表达。事实上,AMPK 缺陷的小鼠胚胎成纤维细胞对苯乙双胍(AMPK 激活剂)和化合物 C(AMPK 抑制剂)没有反应,而雷帕霉素诱导 TXNIP 水平显着增加,证实了已知的 AMPK/mTOR 对 TXNIP 的控制。然而,即使在 AMPK 缺陷的 MEF 中或 mTOR 抑制后,也观察到由 NaCl 或 MGO 诱导的 TXNIP 损失,表明 AMPK/mTOR 不参与这种快速的 TXNIP 损失。这些结果表明,快速的 TXNIP 损失是对压力的一般和即时反应,可以提高能量可用性和抗氧化保护,
更新日期:2019-11-01
中文翻译:

在羰基和高渗应激下,HT22 神经元细胞中 TXNIP 的快速和持续丢失。
硫氧还蛋白相互作用蛋白 (TXNIP) 与硫氧还蛋白结合从而限制其活性,但它也促进葡萄糖转运蛋白的内化,参与炎性体激活并控制自噬。已发表的数据和这项工作表明 TXNIP 对许多明显无关的压力有反应,例如血清剥夺、pH 变化以及氧化、渗透压和羰基压力。有趣的是,我们注意到 HT22 神经元细胞中的高渗(NaCl)和羰基(甲基乙二醛,MGO)应激导致 TXNIP 快速损失(半衰期~12 分钟),促使我们寻找控制这种 TXNIP 损失的可能机制,包括 pH变化、血清剥夺、钙代谢和蛋白酶体和其他蛋白酶的抑制、自噬和 MAPK。这些途径都不能阻止由高渗和羰基胁迫引起的 TXNIP 损失。除了转录、翻译和 microRNA 调节外,有证据表明 mTOR 和 AMPK 也控制 TXNIP 表达。事实上,AMPK 缺陷的小鼠胚胎成纤维细胞对苯乙双胍(AMPK 激活剂)和化合物 C(AMPK 抑制剂)没有反应,而雷帕霉素诱导 TXNIP 水平显着增加,证实了已知的 AMPK/mTOR 对 TXNIP 的控制。然而,即使在 AMPK 缺陷的 MEF 中或 mTOR 抑制后,也观察到由 NaCl 或 MGO 诱导的 TXNIP 损失,表明 AMPK/mTOR 不参与这种快速的 TXNIP 损失。这些结果表明,快速的 TXNIP 损失是对压力的一般和即时反应,可以提高能量可用性和抗氧化保护,











































 京公网安备 11010802027423号
京公网安备 11010802027423号