Stem Cell Reports ( IF 5.9 ) Pub Date : 2019-10-31 , DOI: 10.1016/j.stemcr.2019.10.002 Hyo-Won Han 1 , Hyang-Hee Seo 1 , Hye-Yeong Jo 1 , Hyeong-Jun Han 2 , Virgínia C A Falcão 3 , Vincent Delorme 3 , Jinyeong Heo 4 , David Shum 4 , Jang-Hoon Choi 5 , Jin-Moo Lee 5 , Seung Hun Lee 6 , Hye-Ryeon Heo 7 , Seok-Ho Hong 7 , Mi-Hyun Park 1 , Rajesh K Thimmulappa 8 , Jung-Hyun Kim 1
|
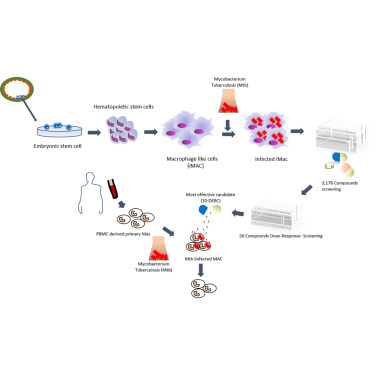
A major limitation in anti-tuberculosis drug screening is the lack of reliable and scalable models for homogeneous human primary macrophage cells of non-cancer origin. Here we report a modified protocol for generating homogeneous populations of macrophage-like cells from human embryonic stem cells. The induced macrophages, referred to as iMACs, presented similar transcriptomic profiles and characteristic immunological features of classical macrophages and were permissive to viral and bacterial infection, in particular Mycobacterium tuberculosis (Mtb). More importantly, iMAC production was amenable to scale up. To evaluate iMAC efficiency in high-throughput anti-tuberculosis drug screening, we performed a phenotypic screening against intracellular Mtb, involving a library of 3,716 compounds that included FDA-approved drugs and other bioactive compounds. Our primary screen identified 120 hits, which were validated in a secondary screen by dose-intracellular and -extracellular Mtb assays. Our confirmatory studies identified a novel anti-Mtb compound, 10-DEBC, also showing activity against drug-resistant strains.
中文翻译:

用人类胚胎干细胞衍生的巨噬细胞靶向结核分枝杆菌的药物发现平台。
抗结核药物筛选的主要局限性在于缺乏可靠且可扩展的非癌症同源人原代巨噬细胞模型。在这里,我们报告从人类胚胎干细胞生成巨噬细胞样细胞的同质群体的修改的协议。诱导的巨噬细胞,称为iMAC,具有类似的转录组谱和经典巨噬细胞的特征性免疫学特征,并允许病毒和细菌感染,特别是结核分枝杆菌感染(MTB)。更重要的是,iMAC的生产可以扩大规模。为了评估iMAC在高通量抗结核药物筛选中的效率,我们针对细胞内Mtb进行了表型筛选,涉及3,716种化合物的文库,其中包括FDA批准的药物和其他生物活性化合物。我们的初步筛选确定了120个点击,通过二次剂量筛选通过细胞内和细胞外Mtb分析进行了验证。我们的验证性研究确定了一种新型抗Mtb化合物10-DEBC,它也显示出对耐药菌株的活性。









































 京公网安备 11010802027423号
京公网安备 11010802027423号