当前位置:
X-MOL 学术
›
Lancet Neurol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Clinical and dopamine transporter imaging characteristics of non-manifest LRRK2 and GBA mutation carriers in the Parkinson's Progression Markers Initiative (PPMI): a cross-sectional study
The Lancet Neurology ( IF 46.5 ) Pub Date : 2020-01-01 , DOI: 10.1016/s1474-4422(19)30319-9 Tanya Simuni 1 , Liz Uribe 2 , Hyunkeun Ryan Cho 2 , Chelsea Caspell-Garcia 2 , Christopher S Coffey 2 , Andrew Siderowf 3 , John Q Trojanowski 4 , Leslie M Shaw 4 , John Seibyl 5 , Andrew Singleton 6 , Arthur W Toga 7 , Doug Galasko 8 , Tatiana Foroud 9 , Duygu Tosun 10 , Kathleen Poston 11 , Daniel Weintraub 12 , Brit Mollenhauer 13 , Caroline M Tanner 10 , Karl Kieburtz 14 , Lana M Chahine 15 , Alyssa Reimer 16 , Samantha J Hutten 16 , Susan Bressman 17 , Kenneth Marek 5 ,
The Lancet Neurology ( IF 46.5 ) Pub Date : 2020-01-01 , DOI: 10.1016/s1474-4422(19)30319-9 Tanya Simuni 1 , Liz Uribe 2 , Hyunkeun Ryan Cho 2 , Chelsea Caspell-Garcia 2 , Christopher S Coffey 2 , Andrew Siderowf 3 , John Q Trojanowski 4 , Leslie M Shaw 4 , John Seibyl 5 , Andrew Singleton 6 , Arthur W Toga 7 , Doug Galasko 8 , Tatiana Foroud 9 , Duygu Tosun 10 , Kathleen Poston 11 , Daniel Weintraub 12 , Brit Mollenhauer 13 , Caroline M Tanner 10 , Karl Kieburtz 14 , Lana M Chahine 15 , Alyssa Reimer 16 , Samantha J Hutten 16 , Susan Bressman 17 , Kenneth Marek 5 ,
Affiliation
|
BACKGROUND
The Parkinson's Progression Markers Initiative (PPMI) is an ongoing observational, longitudinal cohort study of participants with Parkinson's disease, healthy controls, and carriers of the most common Parkinson's disease-related genetic mutations, which aims to define biomarkers of Parkinson's disease diagnosis and progression. All participants are assessed annually with a battery of motor and non-motor scales, 123-I Ioflupane dopamine transporter (DAT) imaging, and biological variables. We aimed to examine whether non-manifesting carriers of LRRK2 and GBA mutations have prodromal features of Parkinson's disease that correlate with reduced DAT binding. METHODS
This cross-sectional analysis is based on assessments done at enrolment in the subset of non-manifesting carriers of LRRK2 and GBA mutations enrolled into the PPMI study from 33 participating sites worldwide. The primary objective was to examine baseline clinical and DAT imaging characteristics in non-manifesting carriers with GBA and LRRK2 mutations compared with healthy controls. DAT deficit was defined as less than 65% of putamen striatal binding ratio expected for the individual's age. We used t tests, χ2 tests, and Fisher's exact tests to compare baseline demographics across groups. An inverse probability weighting method was applied to control for potential confounders such as age and sex. To account for multiple comparisons, we applied a family-wise error rate to each set of analyses. This study is registered with ClinicalTrials.gov, number NCT01141023. FINDINGS
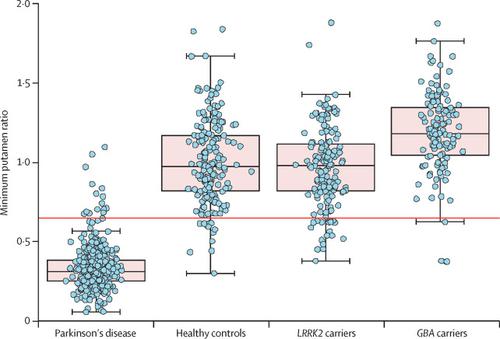
Between Jan 1, 2014, and Jan 1, 2019, the study enrolled 208 LRRK2 (93% G2019S) and 184 GBA (96% N370S) non-manifesting carriers. Both groups were similar with respect to mean age, and about 60% were female. Of the 286 (73%) non-manifesting carriers that had DAT imaging results, 18 (11%) LRRK2 and four (3%) GBA non-manifesting carriers had a DAT deficit. Compared with healthy controls, both LRRK2 and GBA non-manifesting carriers had significantly increased mean scores on the Movement Disorders Society Unified Parkinson's Disease Rating Scale (total score 4·6 [SD 4·4] healthy controls vs 8·4 [7·3] LRRK2 vs 9·5 [9·2] GBA, p<0·0001 for both comparisons) and the Scale for Outcomes for PD - autonomic function (5·8 [3·7] vs 8·1 [5·9] and 8·4 [6·0], p<0·0001 for both comparisons). There was no difference in daytime sleepiness, anxiety, depression, impulsive-compulsive disorders, blood pressure, urate, and rapid eye movement (REM) behaviour disorder scores. Hyposmia was significantly more common only in LRRK2 non-manifesting carriers (69 [36%] of 194 healthy controls vs 114 [55%] of 208 LRRK2 non-manifesting carriers; p=0·0003). Finally, GBA but not LRRK2 non-manifesting carriers showed increased DAT striatal binding ratios compared with healthy controls in the caudate (healthy controls 2·98 [SD 0·63] vs GBA 3·26 [0·63]; p<0·0001), putamen (2·15 [0·56] vs 2·48 [0·52]; p<0·0001), and striatum (2·56 [0·57] vs 2·87 [0·55]; p<0·0001). INTERPRETATION
Our data show evidence of subtle motor and non-motor signs of Parkinson's disease in non-manifesting carriers compared with healthy controls that can precede DAT deficit. Longitudinal data will be essential to confirm these findings and define the trajectory and predictors for development of Parkinson's disease. FUNDING
Michael J Fox Foundation for Parkinson's Research.
中文翻译:

帕金森病进展标志物倡议 (PPMI) 中非明显 LRRK2 和 GBA 突变携带者的临床和多巴胺转运体成像特征:一项横断面研究
背景 帕金森病进展标志物倡议 (PPMI) 是一项针对帕金森病参与者、健康对照者和最常见帕金森病相关基因突变携带者的持续观察性纵向队列研究,旨在确定帕金森病诊断和进展的生物标志物. 所有参与者每年都接受一系列运动和非运动量表、123-I 氟烷多巴胺转运蛋白 (DAT) 成像和生物变量的评估。我们旨在检查 LRRK2 和 GBA 突变的非表现携带者是否具有与 DAT 结合减少相关的帕金森病的前驱特征。方法 该横断面分析基于在全球 33 个参与地点参与 PPMI 研究的 LRRK2 和 GBA 突变非显性携带者子集的注册评估。主要目的是与健康对照相比,检查具有 GBA 和 LRRK2 突变的非表现携带者的基线临床和 DAT 成像特征。DAT 缺陷定义为低于个体年龄预期的壳核纹状体结合率的 65%。我们使用 t 检验、χ2 检验和 Fisher 精确检验来比较各组的基线人口统计学。应用逆概率加权方法来控制潜在的混杂因素,如年龄和性别。为了考虑多重比较,我们对每组分析应用了家庭错误率。本研究已在 ClinicalTrials.gov 注册,编号 NCT01141023。结果 在 2014 年 1 月 1 日至 2019 年 1 月 1 日期间,该研究招募了 208 名 LRRK2(93% G2019S)和 184 名 GBA(96% N370S)未表现出的携带者。两组的平均年龄相似,约 60% 是女性。在具有 DAT 成像结果的 286 (73%) 名无表现携带者中,18 (11%) 名 LRRK2 和四名 (3%) GBA 未表现携带者有 DAT 缺陷。与健康对照相比,LRRK2 和 GBA 非表现携带者在运动障碍协会统一帕金森病评定量表上的平均得分显着增加(总分 4·6 [SD 4·4] 健康对照 vs 8·4 [7·3] ] LRRK2 与 9·5 [9·2] GBA,两个比较的 p<0·0001)和 PD 结果量表 - 自主神经功能(5·8 [3·7] 对 8·1 [5·9])和 8·4 [6·0], p< 0·0001 用于两个比较)。白天嗜睡、焦虑、抑郁、冲动性障碍、血压、尿酸盐和快速眼动 (REM) 行为障碍评分没有差异。嗅觉减退仅在 LRRK2 非表现携带者中更为常见(194 名健康对照者中的 69 [36%] 对 208 名 LRRK2 非表现携带者中的 114 [55%];p=0·0003)。最后,与健康对照相比,GBA 而非 LRRK2 非显性携带者在尾状核中显示出更高的 DAT 纹状体结合率(健康对照 2·98 [SD 0·63] vs GBA 3·26 [0·63];p<0· 0001)、壳核(2·15 [0·56] vs 2·48 [0·52];p<0·0001)和纹状体(2·56 [0·57] vs 2·87 [0·55]) ;p<0·0001)。解释我们的数据显示了帕金森症的细微运动和非运动迹象的证据 与可在 DAT 缺陷之前的健康对照相比,未表现出的携带者中的 s 疾病。纵向数据对于证实这些发现并确定帕金森病发展的轨迹和预测因素至关重要。资助 Michael J Fox 帕金森研究基金会。
更新日期:2020-01-01
中文翻译:

帕金森病进展标志物倡议 (PPMI) 中非明显 LRRK2 和 GBA 突变携带者的临床和多巴胺转运体成像特征:一项横断面研究
背景 帕金森病进展标志物倡议 (PPMI) 是一项针对帕金森病参与者、健康对照者和最常见帕金森病相关基因突变携带者的持续观察性纵向队列研究,旨在确定帕金森病诊断和进展的生物标志物. 所有参与者每年都接受一系列运动和非运动量表、123-I 氟烷多巴胺转运蛋白 (DAT) 成像和生物变量的评估。我们旨在检查 LRRK2 和 GBA 突变的非表现携带者是否具有与 DAT 结合减少相关的帕金森病的前驱特征。方法 该横断面分析基于在全球 33 个参与地点参与 PPMI 研究的 LRRK2 和 GBA 突变非显性携带者子集的注册评估。主要目的是与健康对照相比,检查具有 GBA 和 LRRK2 突变的非表现携带者的基线临床和 DAT 成像特征。DAT 缺陷定义为低于个体年龄预期的壳核纹状体结合率的 65%。我们使用 t 检验、χ2 检验和 Fisher 精确检验来比较各组的基线人口统计学。应用逆概率加权方法来控制潜在的混杂因素,如年龄和性别。为了考虑多重比较,我们对每组分析应用了家庭错误率。本研究已在 ClinicalTrials.gov 注册,编号 NCT01141023。结果 在 2014 年 1 月 1 日至 2019 年 1 月 1 日期间,该研究招募了 208 名 LRRK2(93% G2019S)和 184 名 GBA(96% N370S)未表现出的携带者。两组的平均年龄相似,约 60% 是女性。在具有 DAT 成像结果的 286 (73%) 名无表现携带者中,18 (11%) 名 LRRK2 和四名 (3%) GBA 未表现携带者有 DAT 缺陷。与健康对照相比,LRRK2 和 GBA 非表现携带者在运动障碍协会统一帕金森病评定量表上的平均得分显着增加(总分 4·6 [SD 4·4] 健康对照 vs 8·4 [7·3] ] LRRK2 与 9·5 [9·2] GBA,两个比较的 p<0·0001)和 PD 结果量表 - 自主神经功能(5·8 [3·7] 对 8·1 [5·9])和 8·4 [6·0], p< 0·0001 用于两个比较)。白天嗜睡、焦虑、抑郁、冲动性障碍、血压、尿酸盐和快速眼动 (REM) 行为障碍评分没有差异。嗅觉减退仅在 LRRK2 非表现携带者中更为常见(194 名健康对照者中的 69 [36%] 对 208 名 LRRK2 非表现携带者中的 114 [55%];p=0·0003)。最后,与健康对照相比,GBA 而非 LRRK2 非显性携带者在尾状核中显示出更高的 DAT 纹状体结合率(健康对照 2·98 [SD 0·63] vs GBA 3·26 [0·63];p<0· 0001)、壳核(2·15 [0·56] vs 2·48 [0·52];p<0·0001)和纹状体(2·56 [0·57] vs 2·87 [0·55]) ;p<0·0001)。解释我们的数据显示了帕金森症的细微运动和非运动迹象的证据 与可在 DAT 缺陷之前的健康对照相比,未表现出的携带者中的 s 疾病。纵向数据对于证实这些发现并确定帕金森病发展的轨迹和预测因素至关重要。资助 Michael J Fox 帕金森研究基金会。











































 京公网安备 11010802027423号
京公网安备 11010802027423号