Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study.
The Lancet ( IF 98.4 ) Pub Date : 2019-11-01 , DOI: 10.1016/s0140-6736(19)32591-7 Barbara Burtness , Kevin J Harrington , Richard Greil , Denis Soulières , Makoto Tahara , Gilberto de Castro , Amanda Psyrri , Neus Basté , Prakash Neupane , Åse Bratland , Thorsten Fuereder , Brett G M Hughes , Ricard Mesía , Nuttapong Ngamphaiboon , Tamara Rordorf , Wan Zamaniah Wan Ishak , Ruey-Long Hong , René González Mendoza , Ananya Roy , Yayan Zhang , Burak Gumuscu , Jonathan D Cheng , Fan Jin , Danny Rischin , Guillermo Lerzo , Marcelo Tatangelo , Mirta Varela , Juan Jose Zarba , Michael Boyer , Hui Gan , Bo Gao , Brett Hughes , Girish Mallesara , Danny Rischin , Anne Taylor , Martin Burian , Thorsten Fuereder , Richard Greil , Carlos Henrique Barrios , Dalvaro Oliveira de Castro Junior , Gilberto Castro , Fabio Andre Franke , Gustavo Girotto , Iane Pinto Figueiredo Lima , Ulisses Ribaldo Nicolau , Gustavo Dix Junqueira Pinto , Lucas Santos , Ana-Paula Victorino , Neil Chua , Felix Couture , Richard Gregg , Aaron Hansen , John Hilton , Joy McCarthy , Denis Soulieres , Rodrigo Ascui , Pablo Gonzalez , Luis Villanueva , Marco Torregroza , Angela Zambrano , Petra Holeckova , Zdenek Kral , Bohuslav Melichar , Jana Prausova , Milan Vosmik , Maria Andersen , Niels Gyldenkerne , Hannes Jurgens , Kadri Putnik , Petri Reinikainen , Viktor Gruenwald , Simon Laban , Gerasimos Aravantinos , Ioannis Boukovinas , Vassilis Georgoulias , Amanda Psyrri , Dora Kwong , Yousuf Al-Farhat , Tibor Csoszi , Jozsef Erfan , Geza Horvai , Laszlo Landherr , Eva Remenar , Agnes Ruzsa , Judit Szota , Salem Billan , Iris Gluck , Orit Gutfeld , Aron Popovtzer , Marco Benasso , Simona Bui , Vittorio Ferrari , Lisa Licitra , Franco Nole , Takashi Fujii , Yasushi Fujimoto , Nobuhiro Hanai , Hiroki Hara , Koji Matsumoto , Kenji Mitsugi , Nobuya Monden , Masahiro Nakayama , Kenji Okami , Nobuhiko Oridate , Kiyoto Shiga , Yasushi Shimizu , Masashi Sugasawa , Makoto Tahara , Masanobu Takahashi , Shunji Takahashi , Kaoru Tanaka , Tsutomu Ueda , Hironori Yamaguchi , Tomoko Yamazaki , Ryuji Yasumatsu , Tomoya Yokota , Tomokazu Yoshizaki , Iveta Kudaba , Zinaida Stara , Wan Zamaniah Wan Ishak , Soon Keat Cheah , Jose Aguilar Ponce , Rene Gonzalez Mendoza , Carlos Hernandez Hernandez , Francisco Medina Soto , Jan Buter , Ann Hoeben , S. Oosting , Karijn Suijkerbuijk , Aase Bratland , Marianne Brydoey , Renzo Alvarez , Luis Mas , Priscilla Caguioa , John Querol , Eugenio Emmanuel Regala , Maria Belen Tamayo , Ellie May Villegas , Andrzej Kawecki , Andrey Karpenko , Arkadiy Klochikhin , Alexey Smolin , Oleg Zarubenkov , Boon Cher Goh , Graham Cohen , Johanna du Toit , Christa Jordaan , Gregory Landers , Paul Ruff , Waldemar Szpak , Neonyana Tabane , Irene Brana , Lara Iglesias Docampo , Javier Lavernia , Ricard Mesia , Edvard Abel , Valentina Muratidu , Niels Nielsen , Valerie Cristina , Tamara Rordorf , Sacha Rothschild , Ruey-Long Hong , Hung-Ming Wang , Muh-Hwa Yang , Su-Peng Yeh , Chia-Jui Yen , Nuttapong Ngamphaiboon , Nopadol Soparattanapaisarn , Virote Sriuranpong , Sercan Aksoy , Irfan Cicin , Meltem Ekenel , Hakan Harputluoglu , Ozgur Ozyilkan , Kevin Harrington , Sanjiv Agarwala , Haythem Ali , Robert Alter , Daniel Anderson , Justine Bruce , Barbara Burtness , Nicholas Campbell , Miguel Conde , John Deeken , William Edenfield , Lawrence Feldman , Elizabeth Gaughan , Basem Goueli , Balazs Halmos , Upendra Hegde , Brian Hunis , Robert Jotte , Anand Karnad , Saad Khan , Noel Laudi , Douglas Laux , Danko Martincic , Steven McCune , Dean McGaughey , Krzysztof Misiukiewicz , Deborah Mulford , Eric Nadler , Prakash Neupane , Johannes Nunnink , James Ohr , Meaghan O'Malley , Brian Patson , Doru Paul , Elizabeta Popa , Steven Powell , Rebecca Redman , Vincent Rella , Chaio Rocha Lima , Abirami Sivapiragasam , Yungpo Su , Ammar Sukari , Stuart Wong , Emrullah Yilmaz , Jeffrey Yorio
The Lancet ( IF 98.4 ) Pub Date : 2019-11-01 , DOI: 10.1016/s0140-6736(19)32591-7 Barbara Burtness , Kevin J Harrington , Richard Greil , Denis Soulières , Makoto Tahara , Gilberto de Castro , Amanda Psyrri , Neus Basté , Prakash Neupane , Åse Bratland , Thorsten Fuereder , Brett G M Hughes , Ricard Mesía , Nuttapong Ngamphaiboon , Tamara Rordorf , Wan Zamaniah Wan Ishak , Ruey-Long Hong , René González Mendoza , Ananya Roy , Yayan Zhang , Burak Gumuscu , Jonathan D Cheng , Fan Jin , Danny Rischin , Guillermo Lerzo , Marcelo Tatangelo , Mirta Varela , Juan Jose Zarba , Michael Boyer , Hui Gan , Bo Gao , Brett Hughes , Girish Mallesara , Danny Rischin , Anne Taylor , Martin Burian , Thorsten Fuereder , Richard Greil , Carlos Henrique Barrios , Dalvaro Oliveira de Castro Junior , Gilberto Castro , Fabio Andre Franke , Gustavo Girotto , Iane Pinto Figueiredo Lima , Ulisses Ribaldo Nicolau , Gustavo Dix Junqueira Pinto , Lucas Santos , Ana-Paula Victorino , Neil Chua , Felix Couture , Richard Gregg , Aaron Hansen , John Hilton , Joy McCarthy , Denis Soulieres , Rodrigo Ascui , Pablo Gonzalez , Luis Villanueva , Marco Torregroza , Angela Zambrano , Petra Holeckova , Zdenek Kral , Bohuslav Melichar , Jana Prausova , Milan Vosmik , Maria Andersen , Niels Gyldenkerne , Hannes Jurgens , Kadri Putnik , Petri Reinikainen , Viktor Gruenwald , Simon Laban , Gerasimos Aravantinos , Ioannis Boukovinas , Vassilis Georgoulias , Amanda Psyrri , Dora Kwong , Yousuf Al-Farhat , Tibor Csoszi , Jozsef Erfan , Geza Horvai , Laszlo Landherr , Eva Remenar , Agnes Ruzsa , Judit Szota , Salem Billan , Iris Gluck , Orit Gutfeld , Aron Popovtzer , Marco Benasso , Simona Bui , Vittorio Ferrari , Lisa Licitra , Franco Nole , Takashi Fujii , Yasushi Fujimoto , Nobuhiro Hanai , Hiroki Hara , Koji Matsumoto , Kenji Mitsugi , Nobuya Monden , Masahiro Nakayama , Kenji Okami , Nobuhiko Oridate , Kiyoto Shiga , Yasushi Shimizu , Masashi Sugasawa , Makoto Tahara , Masanobu Takahashi , Shunji Takahashi , Kaoru Tanaka , Tsutomu Ueda , Hironori Yamaguchi , Tomoko Yamazaki , Ryuji Yasumatsu , Tomoya Yokota , Tomokazu Yoshizaki , Iveta Kudaba , Zinaida Stara , Wan Zamaniah Wan Ishak , Soon Keat Cheah , Jose Aguilar Ponce , Rene Gonzalez Mendoza , Carlos Hernandez Hernandez , Francisco Medina Soto , Jan Buter , Ann Hoeben , S. Oosting , Karijn Suijkerbuijk , Aase Bratland , Marianne Brydoey , Renzo Alvarez , Luis Mas , Priscilla Caguioa , John Querol , Eugenio Emmanuel Regala , Maria Belen Tamayo , Ellie May Villegas , Andrzej Kawecki , Andrey Karpenko , Arkadiy Klochikhin , Alexey Smolin , Oleg Zarubenkov , Boon Cher Goh , Graham Cohen , Johanna du Toit , Christa Jordaan , Gregory Landers , Paul Ruff , Waldemar Szpak , Neonyana Tabane , Irene Brana , Lara Iglesias Docampo , Javier Lavernia , Ricard Mesia , Edvard Abel , Valentina Muratidu , Niels Nielsen , Valerie Cristina , Tamara Rordorf , Sacha Rothschild , Ruey-Long Hong , Hung-Ming Wang , Muh-Hwa Yang , Su-Peng Yeh , Chia-Jui Yen , Nuttapong Ngamphaiboon , Nopadol Soparattanapaisarn , Virote Sriuranpong , Sercan Aksoy , Irfan Cicin , Meltem Ekenel , Hakan Harputluoglu , Ozgur Ozyilkan , Kevin Harrington , Sanjiv Agarwala , Haythem Ali , Robert Alter , Daniel Anderson , Justine Bruce , Barbara Burtness , Nicholas Campbell , Miguel Conde , John Deeken , William Edenfield , Lawrence Feldman , Elizabeth Gaughan , Basem Goueli , Balazs Halmos , Upendra Hegde , Brian Hunis , Robert Jotte , Anand Karnad , Saad Khan , Noel Laudi , Douglas Laux , Danko Martincic , Steven McCune , Dean McGaughey , Krzysztof Misiukiewicz , Deborah Mulford , Eric Nadler , Prakash Neupane , Johannes Nunnink , James Ohr , Meaghan O'Malley , Brian Patson , Doru Paul , Elizabeta Popa , Steven Powell , Rebecca Redman , Vincent Rella , Chaio Rocha Lima , Abirami Sivapiragasam , Yungpo Su , Ammar Sukari , Stuart Wong , Emrullah Yilmaz , Jeffrey Yorio
|
BACKGROUND
Pembrolizumab is active in head and neck squamous cell carcinoma (HNSCC), with programmed cell death ligand 1 (PD-L1) expression associated with improved response.
METHODS
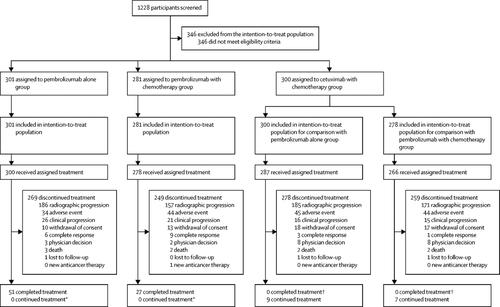
KEYNOTE-048 was a randomised, phase 3 study of participants with untreated locally incurable recurrent or metastatic HNSCC done at 200 sites in 37 countries. Participants were stratified by PD-L1 expression, p16 status, and performance status and randomly allocated (1:1:1) to pembrolizumab alone, pembrolizumab plus a platinum and 5-fluorouracil (pembrolizumab with chemotherapy), or cetuximab plus a platinum and 5-fluorouracil (cetuximab with chemotherapy). Investigators and participants were aware of treatment assignment. Investigators, participants, and representatives of the sponsor were masked to the PD-L1 combined positive score (CPS) results; PD-L1 positivity was not required for study entry. The primary endpoints were overall survival (time from randomisation to death from any cause) and progression-free survival (time from randomisation to radiographically confirmed disease progression or death from any cause, whichever came first) in the intention-to-treat population (all participants randomly allocated to a treatment group). There were 14 primary hypotheses: superiority of pembrolizumab alone and of pembrolizumab with chemotherapy versus cetuximab with chemotherapy for overall survival and progression-free survival in the PD-L1 CPS of 20 or more, CPS of 1 or more, and total populations and non-inferiority (non-inferiority margin: 1·2) of pembrolizumab alone and pembrolizumab with chemotherapy versus cetuximab with chemotherapy for overall survival in the total population. The definitive findings for each hypothesis were obtained when statistical testing was completed for that hypothesis; this occurred at the second interim analysis for 11 hypotheses and at final analysis for three hypotheses. Safety was assessed in the as-treated population (all participants who received at least one dose of allocated treatment). This study is registered at ClinicalTrials.gov, number NCT02358031.
FINDINGS
Between April 20, 2015, and Jan 17, 2017, 882 participants were allocated to receive pembrolizumab alone (n=301), pembrolizumab with chemotherapy (n=281), or cetuximab with chemotherapy (n=300); of these, 754 (85%) had CPS of 1 or more and 381 (43%) had CPS of 20 or more. At the second interim analysis, pembrolizumab alone improved overall survival versus cetuximab with chemotherapy in the CPS of 20 or more population (median 14·9 months vs 10·7 months, hazard ratio [HR] 0·61 [95% CI 0·45-0·83], p=0·0007) and CPS of 1 or more population (12·3 vs 10·3, 0·78 [0·64-0·96], p=0·0086) and was non-inferior in the total population (11·6 vs 10·7, 0·85 [0·71-1·03]). Pembrolizumab with chemotherapy improved overall survival versus cetuximab with chemotherapy in the total population (13·0 months vs 10·7 months, HR 0·77 [95% CI 0·63-0·93], p=0·0034) at the second interim analysis and in the CPS of 20 or more population (14·7 vs 11·0, 0·60 [0·45-0·82], p=0·0004) and CPS of 1 or more population (13·6 vs 10·4, 0·65 [0·53-0·80], p<0·0001) at final analysis. Neither pembrolizumab alone nor pembrolizumab with chemotherapy improved progression-free survival at the second interim analysis. At final analysis, grade 3 or worse all-cause adverse events occurred in 164 (55%) of 300 treated participants in the pembrolizumab alone group, 235 (85%) of 276 in the pembrolizumab with chemotherapy group, and 239 (83%) of 287 in the cetuximab with chemotherapy group. Adverse events led to death in 25 (8%) participants in the pembrolizumab alone group, 32 (12%) in the pembrolizumab with chemotherapy group, and 28 (10%) in the cetuximab with chemotherapy group.
INTERPRETATION
Based on the observed efficacy and safety, pembrolizumab plus platinum and 5-fluorouracil is an appropriate first-line treatment for recurrent or metastatic HNSCC and pembrolizumab monotherapy is an appropriate first-line treatment for PD-L1-positive recurrent or metastatic HNSCC.
FUNDING
Merck Sharp & Dohme.
中文翻译:

单用派姆单抗或化疗与西妥昔单抗联合化疗对头颈部复发或转移性鳞状细胞癌的治疗(KEYNOTE-048):一项随机,开放标签的3期研究。
背景派姆单抗在头颈部鳞状细胞癌(HNSCC)中活跃,其程序性细胞死亡配体1(PD-L1)表达与改善的反应相关。方法KEYNOTE-048是一项随机的,3期研究,研究对象是在37个国家/地区的200个地点进行的未经治疗的局部不可治愈的复发性或转移性HNSCC参与者。参与者按PD-L1表达,p16状态和表现状态进行分层,并随机分配(1:1:1)单独使用pembrolizumab,pembrolizumab加铂和5-氟尿嘧啶(pembrolizumab化疗)或西妥昔单抗加铂和5 -氟尿嘧啶(西妥昔单抗联合化疗)。研究者和参与者都知道治疗分配。研究人员,参与者和发起人的代表对PD-L1综合阳性评分(CPS)结果不了解;进入研究不需要PD-L1阳性。主要终点是意向性治疗人群(全部)的总体生存期(从随机分组到因任何原因死亡的时间)和无进展生存期(从随机分组到通过射线照相确认的疾病进展或因任何原因死亡的时间,以先到者为准)。参与者随机分配到一个治疗组)。有14个主要假设:PD-L1的CPS为20或更高,CPS为1或更高,总人群和非人群,单独使用pembrolizumab和使用pembrolizumab联合化疗比使用cetuximab联合化疗的总生存期和无进展生存期优越。在总人群中,单独使用pembrolizumab和使用pembrolizumab联合化疗与使用西妥昔单抗联合化疗相比,使用pembrolizumab的自卑程度(非自卑程度:1·2)。当每个假设的统计检验完成后,便获得了每个假设的确定性发现。这发生在针对11个假设的第二次中期分析和针对三个假设的最终分析中。在接受治疗的人群(接受至少一剂指定治疗剂量的所有参与者)中评估安全性。该研究已在ClinicalTrials.gov上注册,编号为NCT02358031。结果在2015年4月20日至2017年1月17日之间,分配了882名参与者单独接受派姆单抗(n = 301),接受化疗的派姆单抗(n = 281)或接受化疗的西妥昔单抗(n = 300);其中754(85%)的CPS为1或更高,而381(43%)的CPS为20或更高。在第二次中期分析中,在20个或更多的人群中,单独使用pembrolizumab与西妥昔单抗联合化疗可改善总体生存率(中位14·9个月vs 10·7个月,危险比[HR] 0·61 [95%CI 0·45-0·83], p = 0·0007)和1个或更多人群的CPS(12·3 vs 10·3,0·78 [0·64-0·96],p = 0·0086),在总人群中不逊色(11·6 vs 10·7,0·85 [0·71-1·03])。与西妥昔单抗联合化疗相比,在所有人群中接受化疗的派姆单抗改善了总生存期(13·0个月vs 10·7个月,HR 0·77 [95%CI 0·63-0·93],p = 0·0034)。第二次中期分析和20个或更多人口的CPS(14·7对11·0、0·60 [0·45-0·82],p = 0·0004)和1个或更多人口的CPS(13·最终分析显示6 vs 10·4,0·65 [0·53-0·80],p <0·0001)。在第二次中期分析中,单独使用pembrolizumab或使用化疗的pembrolizumab均不能改善无进展生存期。归根结底,在单独使用派姆单抗的300名接受治疗的参与者中,有164名(55%)发生了3级或更严重的全因不良事件,在使用化疗的派姆单抗的276名患者中,有235名(85%)发生了化疗,还有239名(83%)西妥昔单抗联合化疗组287例患者。不良事件导致单独使用pembrolizumab组的25名患者(8%)死亡,使用化疗组的pembrolizumab组32名(12%)死亡,使用化疗组的西妥昔单抗28名(10%)死亡。解释根据观察到的疗效和安全性,派姆单抗加铂和5-氟尿嘧啶是复发或转移性HNSCC的合适的一线治疗,派姆单抗单药治疗是PD-L1阳性复发或转移性HNSCC的合适的一线治疗。资金来源默克·夏普&Dohme。
更新日期:2019-11-22
中文翻译:

单用派姆单抗或化疗与西妥昔单抗联合化疗对头颈部复发或转移性鳞状细胞癌的治疗(KEYNOTE-048):一项随机,开放标签的3期研究。
背景派姆单抗在头颈部鳞状细胞癌(HNSCC)中活跃,其程序性细胞死亡配体1(PD-L1)表达与改善的反应相关。方法KEYNOTE-048是一项随机的,3期研究,研究对象是在37个国家/地区的200个地点进行的未经治疗的局部不可治愈的复发性或转移性HNSCC参与者。参与者按PD-L1表达,p16状态和表现状态进行分层,并随机分配(1:1:1)单独使用pembrolizumab,pembrolizumab加铂和5-氟尿嘧啶(pembrolizumab化疗)或西妥昔单抗加铂和5 -氟尿嘧啶(西妥昔单抗联合化疗)。研究者和参与者都知道治疗分配。研究人员,参与者和发起人的代表对PD-L1综合阳性评分(CPS)结果不了解;进入研究不需要PD-L1阳性。主要终点是意向性治疗人群(全部)的总体生存期(从随机分组到因任何原因死亡的时间)和无进展生存期(从随机分组到通过射线照相确认的疾病进展或因任何原因死亡的时间,以先到者为准)。参与者随机分配到一个治疗组)。有14个主要假设:PD-L1的CPS为20或更高,CPS为1或更高,总人群和非人群,单独使用pembrolizumab和使用pembrolizumab联合化疗比使用cetuximab联合化疗的总生存期和无进展生存期优越。在总人群中,单独使用pembrolizumab和使用pembrolizumab联合化疗与使用西妥昔单抗联合化疗相比,使用pembrolizumab的自卑程度(非自卑程度:1·2)。当每个假设的统计检验完成后,便获得了每个假设的确定性发现。这发生在针对11个假设的第二次中期分析和针对三个假设的最终分析中。在接受治疗的人群(接受至少一剂指定治疗剂量的所有参与者)中评估安全性。该研究已在ClinicalTrials.gov上注册,编号为NCT02358031。结果在2015年4月20日至2017年1月17日之间,分配了882名参与者单独接受派姆单抗(n = 301),接受化疗的派姆单抗(n = 281)或接受化疗的西妥昔单抗(n = 300);其中754(85%)的CPS为1或更高,而381(43%)的CPS为20或更高。在第二次中期分析中,在20个或更多的人群中,单独使用pembrolizumab与西妥昔单抗联合化疗可改善总体生存率(中位14·9个月vs 10·7个月,危险比[HR] 0·61 [95%CI 0·45-0·83], p = 0·0007)和1个或更多人群的CPS(12·3 vs 10·3,0·78 [0·64-0·96],p = 0·0086),在总人群中不逊色(11·6 vs 10·7,0·85 [0·71-1·03])。与西妥昔单抗联合化疗相比,在所有人群中接受化疗的派姆单抗改善了总生存期(13·0个月vs 10·7个月,HR 0·77 [95%CI 0·63-0·93],p = 0·0034)。第二次中期分析和20个或更多人口的CPS(14·7对11·0、0·60 [0·45-0·82],p = 0·0004)和1个或更多人口的CPS(13·最终分析显示6 vs 10·4,0·65 [0·53-0·80],p <0·0001)。在第二次中期分析中,单独使用pembrolizumab或使用化疗的pembrolizumab均不能改善无进展生存期。归根结底,在单独使用派姆单抗的300名接受治疗的参与者中,有164名(55%)发生了3级或更严重的全因不良事件,在使用化疗的派姆单抗的276名患者中,有235名(85%)发生了化疗,还有239名(83%)西妥昔单抗联合化疗组287例患者。不良事件导致单独使用pembrolizumab组的25名患者(8%)死亡,使用化疗组的pembrolizumab组32名(12%)死亡,使用化疗组的西妥昔单抗28名(10%)死亡。解释根据观察到的疗效和安全性,派姆单抗加铂和5-氟尿嘧啶是复发或转移性HNSCC的合适的一线治疗,派姆单抗单药治疗是PD-L1阳性复发或转移性HNSCC的合适的一线治疗。资金来源默克·夏普&Dohme。











































 京公网安备 11010802027423号
京公网安备 11010802027423号