当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of All-Carbon Quaternary Centers by Palladium-Catalyzed Olefin Dicarbofunctionalization.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-10-31 , DOI: 10.1002/anie.201911012 Maximilian Koy 1 , Peter Bellotti 1 , Felix Katzenburg 1 , Constantin G Daniliuc 1 , Frank Glorius 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-10-31 , DOI: 10.1002/anie.201911012 Maximilian Koy 1 , Peter Bellotti 1 , Felix Katzenburg 1 , Constantin G Daniliuc 1 , Frank Glorius 1
Affiliation
|
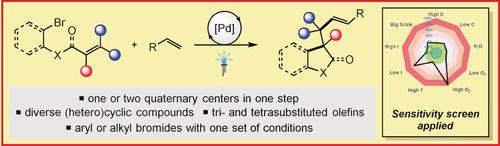
The redox-neutral dicarbofunctionalization of tri- and tetrasubstituted olefins to form a variety of (hetero)cyclic compounds under photoinduced palladium catalysis is described. This cascade reaction process was used to couple styrenes or acryl amides with a broad range of highly decorated olefins tethered to aryl or alkyl bromides (>50 examples). This procedure enables one or two contiguous all-carbon quaternary centers to be formed in a single step. The products could be readily diversified and applied in the synthesis of a bioactive oxindole analogue.
中文翻译:

通过钯催化的烯烃双碳官能化合成全碳四元中心。
描述了在光诱导的钯催化下三和四取代的烯烃的氧化还原-中性二碳官能化以形成各种(杂环)环化合物。该级联反应过程用于将苯乙烯或丙烯酰胺与广泛束缚在芳基或烷基溴上的高度装饰的烯烃偶联(> 50个实例)。此过程使一个或两个连续的全碳四元中心可以在一个步骤中形成。产物可以容易地多样化并用于生物活性羟吲哚类似物的合成。
更新日期:2020-01-10
中文翻译:

通过钯催化的烯烃双碳官能化合成全碳四元中心。
描述了在光诱导的钯催化下三和四取代的烯烃的氧化还原-中性二碳官能化以形成各种(杂环)环化合物。该级联反应过程用于将苯乙烯或丙烯酰胺与广泛束缚在芳基或烷基溴上的高度装饰的烯烃偶联(> 50个实例)。此过程使一个或两个连续的全碳四元中心可以在一个步骤中形成。产物可以容易地多样化并用于生物活性羟吲哚类似物的合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号