当前位置:
X-MOL 学术
›
Surf. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Room Temperature Oxidation of GaAs(110) Using High Translational Kinetic Energy Molecular Beams of O2 Visualized by STM
Surface Science ( IF 2.1 ) Pub Date : 2020-02-01 , DOI: 10.1016/j.susc.2019.121516 Tim Grabnic , Ross Edel , S.J. Sibener
Surface Science ( IF 2.1 ) Pub Date : 2020-02-01 , DOI: 10.1016/j.susc.2019.121516 Tim Grabnic , Ross Edel , S.J. Sibener
|
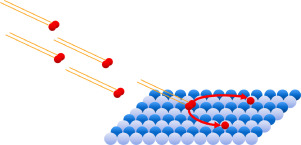
Abstract This study examines the reactive surface dynamics of GaAs(110) oxidation with molecular oxygen at room temperature over a range of impinging kinetic energies. Visualization of the surface by scanning tunneling microscopy (STM) after exposures to O2 with kinetic energies of 0.4–1.2 eV provides morphological and kinetic data that were obtained utilizing a novel instrument that combines a supersonic molecular beam with an in-line, in-situ STM. Oxidation was found to proceed by two morphologically distinct, competing mechanisms: a spatially homogeneous process with randomly distributed chemisorbed oxygen atoms leading to layer-by-layer oxide growth, and a spatially heterogeneous process with oxides nucleating on structural surface defects and growing vertically and laterally with continued exposure. Both oxidation mechanisms exhibit enhanced reactivity with increasing kinetic energy. Only trace oxidation was observed with O2 kinetic energies below 0.7 eV; a rapid increase in the rate of oxidation from 1.0 to 1.2 eV was found with homogeneous and heterogeneous oxidation proceeding simultaneously until full surface coverage was reached. In addition, the relative rates of the two mechanisms appear to change with O2 kinetic energy: spatially homogeneous oxidation is expected to dominate at lower kinetic energies (
中文翻译:

使用 STM 可视化的 O2 高平移动能分子束对 GaAs(110) 进行室温氧化
摘要 本研究研究了 GaAs(110) 在室温下与分子氧在一定范围的撞击动能下氧化的反应性表面动力学。暴露于动能为 0.4-1.2 eV 的 O2 后,通过扫描隧道显微镜 (STM) 对表面进行可视化,提供形态学和动力学数据,这些数据是利用一种将超音速分子束与在线原位相结合的新型仪器获得的STM。发现氧化通过两种形态不同的竞争机制进行:随机分布的化学吸附氧原子导致逐层氧化物生长的空间均质过程,以及氧化物在结构表面缺陷上成核并垂直和横向生长的空间异质过程随着持续曝光。随着动能的增加,两种氧化机制都表现出增强的反应性。当 O2 动能低于 0.7 eV 时,仅观察到微量氧化;发现氧化速率从 1.0 eV 迅速增加到 1.2 eV,同质和异质氧化同时进行,直到达到全表面覆盖。此外,这两种机制的相对速率似乎随着 O2 动能而变化:空间均匀氧化预计在较低动能下占主导地位(
更新日期:2020-02-01
中文翻译:

使用 STM 可视化的 O2 高平移动能分子束对 GaAs(110) 进行室温氧化
摘要 本研究研究了 GaAs(110) 在室温下与分子氧在一定范围的撞击动能下氧化的反应性表面动力学。暴露于动能为 0.4-1.2 eV 的 O2 后,通过扫描隧道显微镜 (STM) 对表面进行可视化,提供形态学和动力学数据,这些数据是利用一种将超音速分子束与在线原位相结合的新型仪器获得的STM。发现氧化通过两种形态不同的竞争机制进行:随机分布的化学吸附氧原子导致逐层氧化物生长的空间均质过程,以及氧化物在结构表面缺陷上成核并垂直和横向生长的空间异质过程随着持续曝光。随着动能的增加,两种氧化机制都表现出增强的反应性。当 O2 动能低于 0.7 eV 时,仅观察到微量氧化;发现氧化速率从 1.0 eV 迅速增加到 1.2 eV,同质和异质氧化同时进行,直到达到全表面覆盖。此外,这两种机制的相对速率似乎随着 O2 动能而变化:空间均匀氧化预计在较低动能下占主导地位(











































 京公网安备 11010802027423号
京公网安备 11010802027423号