当前位置:
X-MOL 学术
›
BBA Proteins Proteom.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
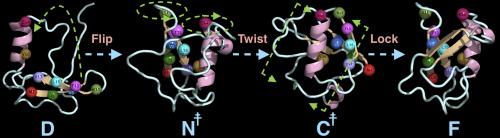
Ubiquitin folds via a flip-twist-lock mechanism.
Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics ( IF 2.5 ) Pub Date : 2019-10-30 , DOI: 10.1016/j.bbapap.2019.140299 Manoj Mandal 1 , Atanu Das 2 , Chaitali Mukhopadhyay 2
Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics ( IF 2.5 ) Pub Date : 2019-10-30 , DOI: 10.1016/j.bbapap.2019.140299 Manoj Mandal 1 , Atanu Das 2 , Chaitali Mukhopadhyay 2
Affiliation
|
To perform specific functional activities, the majority of proteins should fold into their distinct three-dimensional conformations. However, the biologically active conformation of a protein is generally found to be marginally stable than the other conformations that the chain can adopt. How a protein finds its native conformation from its post-synthesis unfolded structure in a complex conformational landscape is the unsolved question that still drives the protein folding community. Here, we report the folding mechanism of a globular protein, ubiquitin, from its chemically denatured state using all-atom molecular dynamics simulations. From the kinetic analysis of the simulated trajectories we show that the folding process can be described by the hydrophobic collapse mechanism, initiated by the "dewetting transition", and subsequently assisted by the origination of an N-terminal folding nucleus, and finally supported by a native salt-bridge interaction between K11 and E34. We show that ubiquitin folds via an intermediate. Finally, we confirm the presence of "biological water" and explain its role to the folding process.
中文翻译:

遍在蛋白通过翻转扭锁机制折叠。
为了执行特定的功能活性,大多数蛋白质应折叠成其独特的三维构象。但是,通常发现蛋白质的生物活性构象比链可以采用的其他构象略微稳定。蛋白质如何从其合成后的未折叠结构在复杂的构象景观中找到其天然构象是尚未解决的问题,仍然驱动着蛋白质折叠群落。在这里,我们使用全原子分子动力学模拟从其化学变性状态报告球蛋白泛素的折叠机制。从模拟轨迹的动力学分析中,我们可以看出,折叠过程可以通过疏水性塌陷机理来描述,而疏水塌陷机理是由“去湿转变”引发的,随后由N末端折叠核的起源协助,最后由K11和E34之间的天然盐桥相互作用所支持。我们显示泛素通过中间体折叠。最后,我们确认“生物水”的存在并解释其在折叠过程中的作用。
更新日期:2019-10-30
中文翻译:

遍在蛋白通过翻转扭锁机制折叠。
为了执行特定的功能活性,大多数蛋白质应折叠成其独特的三维构象。但是,通常发现蛋白质的生物活性构象比链可以采用的其他构象略微稳定。蛋白质如何从其合成后的未折叠结构在复杂的构象景观中找到其天然构象是尚未解决的问题,仍然驱动着蛋白质折叠群落。在这里,我们使用全原子分子动力学模拟从其化学变性状态报告球蛋白泛素的折叠机制。从模拟轨迹的动力学分析中,我们可以看出,折叠过程可以通过疏水性塌陷机理来描述,而疏水塌陷机理是由“去湿转变”引发的,随后由N末端折叠核的起源协助,最后由K11和E34之间的天然盐桥相互作用所支持。我们显示泛素通过中间体折叠。最后,我们确认“生物水”的存在并解释其在折叠过程中的作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号