当前位置:
X-MOL 学术
›
BBA Mol. Cell Biol. Lipids
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
PPARA/RXRA signalling regulates the fate of hepatic non-esterified fatty acids in a sheep model of maternal undernutrition.
Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids ( IF 3.9 ) Pub Date : 2019-10-30 , DOI: 10.1016/j.bbalip.2019.158548 Yanfeng Xue 1 , Changzheng Guo 1 , Fan Hu 1 , Weiyun Zhu 1 , Shengyong Mao 1
Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids ( IF 3.9 ) Pub Date : 2019-10-30 , DOI: 10.1016/j.bbalip.2019.158548 Yanfeng Xue 1 , Changzheng Guo 1 , Fan Hu 1 , Weiyun Zhu 1 , Shengyong Mao 1
Affiliation
|
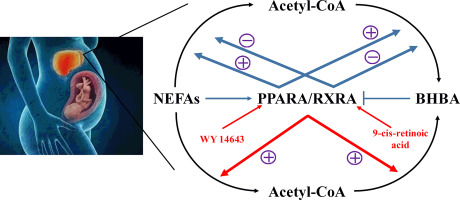
Maternal undernutrition during late gestation accelerates body fat mobilization to provide more energy for foetal growth and development, which unbalances metabolic homeostasis and results in serious lipid metabolism disorder. However, detailed regulatory mechanisms are poorly understood. Here, a sheep model was used to explore the regulatory role of PPARA/RXRA signalling in hepatic lipid metabolism in undernutrition based on RNA sequencing and cell experiments. KOG function classification showed that lipid transport and metabolism was markedly altered in an undernourished model. In detail, when compared with the controls, fatty acid transport and oxidation and triglyceride metabolism were up-regulated in an undernourished model, while fatty acid synthesis, steroid synthesis, and phospholipid metabolism were down-regulated. Kyoto Encyclopaedia of Genes and Genomes (KEGG) pathway analysis demonstrated that PPARA/RXRA signalling pathway was altered. Moreover, PPARA signalling associated genes were positively correlated with hepatic non-esterified fatty acid (NEFA) levels, while retinol metabolism associated genes were negatively correlated with blood beta-hydroxybutyric acid (BHBA) levels. Results of primary hepatocytes showed that NEFAs could activate PPARA signalling and facilitate fatty acid oxidation (FAO) and ketogenesis, while BHBA could inhibit RXRA signalling and repress FAO and ketogenesis. Excessively accumulated NEFAs in hepatocytes promoted triglyceride synthesis. Furthermore, activation of PPARA/RXRA signalling by WY14643 and 9-cis-retinoic acid could enhance FAO and ketogenesis and reduce NEFAs accumulation and esterification. Our findings elucidate the regulatory mechanisms of NEFAs and BHBA on lipid metabolism as well as the potential role of the PPARA/RXRA signalling pathway in hepatic lipid metabolism, which may contribute to exploring new strategies to maintain lipid metabolic homeostasis in human beings.
中文翻译:

PPARA / RXRA信号传导调节母体营养不良的绵羊模型中肝脏非酯化脂肪酸的命运。
孕晚期孕妇的营养不良会加速体内脂肪的动员,从而为胎儿的生长和发育提供更多的能量,从而使代谢体内的平衡失衡,并导致严重的脂质代谢紊乱。但是,人们对详细的监管机制知之甚少。在这里,基于RNA测序和细胞实验,使用绵羊模型来研究PPARA / RXRA信号在营养不足的肝脏脂质代谢中的调节作用。KOG功能分类显示,在营养不良的模型中,脂质转运和代谢显着改变。详细地,当与对照相比时,在营养不足的模型中脂肪酸运输和氧化以及甘油三酸酯代谢被上调,而脂肪酸合成,类固醇合成和磷脂代谢被下调。京都基因与基因组百科全书(KEGG)途径分析表明,PPARA / RXRA信号传导途径已被改变。此外,PPARA信号相关基因与肝非酯化脂肪酸(NEFA)水平呈正相关,而视黄醇代谢相关基因与血液β-羟基丁酸(BHBA)水平呈负相关。原代肝细胞的结果表明,NEFAs可以激活PPARA信号传导并促进脂肪酸氧化(FAO)和生酮作用,而BHBA可以抑制RXRA信号传导并抑制FAO和生酮作用。肝细胞中过度积累的NEFA促进了甘油三酸酯的合成。此外,WY14643和9-顺式-视黄酸激活PPARA / RXRA信号传导可增强FAO和生酮作用,并减少NEFA的积累和酯化。
更新日期:2019-10-30
中文翻译:

PPARA / RXRA信号传导调节母体营养不良的绵羊模型中肝脏非酯化脂肪酸的命运。
孕晚期孕妇的营养不良会加速体内脂肪的动员,从而为胎儿的生长和发育提供更多的能量,从而使代谢体内的平衡失衡,并导致严重的脂质代谢紊乱。但是,人们对详细的监管机制知之甚少。在这里,基于RNA测序和细胞实验,使用绵羊模型来研究PPARA / RXRA信号在营养不足的肝脏脂质代谢中的调节作用。KOG功能分类显示,在营养不良的模型中,脂质转运和代谢显着改变。详细地,当与对照相比时,在营养不足的模型中脂肪酸运输和氧化以及甘油三酸酯代谢被上调,而脂肪酸合成,类固醇合成和磷脂代谢被下调。京都基因与基因组百科全书(KEGG)途径分析表明,PPARA / RXRA信号传导途径已被改变。此外,PPARA信号相关基因与肝非酯化脂肪酸(NEFA)水平呈正相关,而视黄醇代谢相关基因与血液β-羟基丁酸(BHBA)水平呈负相关。原代肝细胞的结果表明,NEFAs可以激活PPARA信号传导并促进脂肪酸氧化(FAO)和生酮作用,而BHBA可以抑制RXRA信号传导并抑制FAO和生酮作用。肝细胞中过度积累的NEFA促进了甘油三酸酯的合成。此外,WY14643和9-顺式-视黄酸激活PPARA / RXRA信号传导可增强FAO和生酮作用,并减少NEFA的积累和酯化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号