当前位置:
X-MOL 学术
›
BBA Bioenerg.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fourier transform infrared and mass spectrometry analyses of a site-directed mutant of D1-Asp170 as a ligand to the water-oxidizing Mn4CaO5 cluster in photosystem II.
Biochimica et Biophysica Acta (BBA) - Bioenergetics ( IF 3.4 ) Pub Date : 2019-10-31 , DOI: 10.1016/j.bbabio.2019.148086 Tomomi Kitajima-Ihara 1 , Takehiro Suzuki 2 , Shin Nakamura 1 , Yuichiro Shimada 1 , Ryo Nagao 1 , Naoshi Dohmae 2 , Takumi Noguchi 1
Biochimica et Biophysica Acta (BBA) - Bioenergetics ( IF 3.4 ) Pub Date : 2019-10-31 , DOI: 10.1016/j.bbabio.2019.148086 Tomomi Kitajima-Ihara 1 , Takehiro Suzuki 2 , Shin Nakamura 1 , Yuichiro Shimada 1 , Ryo Nagao 1 , Naoshi Dohmae 2 , Takumi Noguchi 1
Affiliation
|
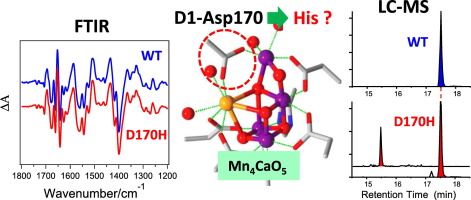
The Mn4CaO5 cluster, the catalytic center of water oxidation in photosystem II (PSII), is coordinated by six carboxylate and one imidazole ligands. The roles of these ligands in the water oxidation mechanism remain largely unknown. In this study, we constructed a D1-D170H mutant, in which the Asp ligand bridging Mn and Ca ions was replaced with His, in the cyanobacterium Synechocystis sp. PCC 6803, and analyzed isolated PSII core complexes using Fourier transform infrared (FTIR) difference spectroscopy and mass spectrometry (MS). The S2-minus-S1 FTIR difference spectrum of the PSII complexes of the D1-D170H mutant showed features virtually identical to those of the wild-type PSII. MS analysis further showed that ~70% of D1 proteins from the PSII complexes of D1-D170H possessed the wild-type amino acid sequence, although only the mutated sequence was detected in genomic DNA in the same batch of cells for PSII preparations. In contrast, a D1-S169A mutant as a control showed a modified FTIR spectrum and only a mutated D1 protein. It is thus concluded that the FTIR spectrum of the D1-D170H mutant actually reflects that of wild-type PSII, whereas the Mn4CaO5 cluster is not formed in PSII with D1-D170H mutation. Although the mechanism of production of the wild-type D1 protein in the D1-D170H mutant is unknown at present, a caution is necessary in the analysis of site-directed mutants of crucial residues in the D1 protein, and mutation has to be confirmed not only at the DNA level but also at the amino acid level.
中文翻译:

D1-Asp170定点突变体的傅里叶变换红外和质谱分析,该突变体是光系统II中水氧化Mn4CaO5簇的配体。
Mn4CaO5簇是光系统II(PSII)中水氧化的催化中心,由6个羧酸盐和1个咪唑配体协调。这些配体在水氧化机理中的作用仍然未知。在这项研究中,我们构建了一个D1-D170H突变体,其中蓝藻Synechocystis sp。中桥接Mn和Ca离子的Asp配体被His取代。PCC 6803,并使用傅立叶变换红外(FTIR)差光谱和质谱(MS)分析了分离的PSII核心复合物。D1-D170H突变体的PSII复合物的S2-负S1 FTIR差异光谱显示出与野生型PSII几乎相同的特征。质谱分析进一步表明,来自D1-D170H的PSII复合物的D1蛋白中约有70%具有野生型氨基酸序列,尽管在用于PSII制备的同一批细胞中仅在基因组DNA中检测到突变序列。相反,作为对照的D1-S169A突变体显示出改良的FTIR光谱,仅显示了突变的D1蛋白。因此可以得出结论,D1-D170H突变体的FTIR谱实际上反映了野生型PSII的FTIR谱,而在具有D1-D170H突变的PSII中没有形成Mn4CaO5簇。尽管目前尚不清楚D1-D170H突变体中野生型D1蛋白的产生机理,但在分析D1蛋白中关键残基的定点突变体时必须谨慎,并且必须确认突变不仅在DNA水平,而且在氨基酸水平。作为对照的D1-S169A突变体显示出改良的FTIR光谱,仅显示了突变的D1蛋白。因此可以得出结论,D1-D170H突变体的FTIR谱实际上反映了野生型PSII的FTIR谱,而在具有D1-D170H突变的PSII中没有形成Mn4CaO5簇。尽管目前尚不清楚D1-D170H突变体中野生型D1蛋白的产生机理,但在分析D1蛋白中关键残基的定点突变体时必须谨慎,并且必须确认突变不仅在DNA水平,而且在氨基酸水平。作为对照的D1-S169A突变体显示出改良的FTIR光谱,仅显示了突变的D1蛋白。因此可以得出结论,D1-D170H突变体的FTIR光谱实际上反映了野生型PSII的FTIR光谱,而在具有D1-D170H突变的PSII中没有形成Mn4CaO5簇。尽管目前尚不清楚D1-D170H突变体中野生型D1蛋白的产生机理,但在分析D1蛋白中关键残基的定点突变体时必须谨慎,并且必须确认突变不仅在DNA水平,而且在氨基酸水平。
更新日期:2019-11-01
中文翻译:

D1-Asp170定点突变体的傅里叶变换红外和质谱分析,该突变体是光系统II中水氧化Mn4CaO5簇的配体。
Mn4CaO5簇是光系统II(PSII)中水氧化的催化中心,由6个羧酸盐和1个咪唑配体协调。这些配体在水氧化机理中的作用仍然未知。在这项研究中,我们构建了一个D1-D170H突变体,其中蓝藻Synechocystis sp。中桥接Mn和Ca离子的Asp配体被His取代。PCC 6803,并使用傅立叶变换红外(FTIR)差光谱和质谱(MS)分析了分离的PSII核心复合物。D1-D170H突变体的PSII复合物的S2-负S1 FTIR差异光谱显示出与野生型PSII几乎相同的特征。质谱分析进一步表明,来自D1-D170H的PSII复合物的D1蛋白中约有70%具有野生型氨基酸序列,尽管在用于PSII制备的同一批细胞中仅在基因组DNA中检测到突变序列。相反,作为对照的D1-S169A突变体显示出改良的FTIR光谱,仅显示了突变的D1蛋白。因此可以得出结论,D1-D170H突变体的FTIR谱实际上反映了野生型PSII的FTIR谱,而在具有D1-D170H突变的PSII中没有形成Mn4CaO5簇。尽管目前尚不清楚D1-D170H突变体中野生型D1蛋白的产生机理,但在分析D1蛋白中关键残基的定点突变体时必须谨慎,并且必须确认突变不仅在DNA水平,而且在氨基酸水平。作为对照的D1-S169A突变体显示出改良的FTIR光谱,仅显示了突变的D1蛋白。因此可以得出结论,D1-D170H突变体的FTIR谱实际上反映了野生型PSII的FTIR谱,而在具有D1-D170H突变的PSII中没有形成Mn4CaO5簇。尽管目前尚不清楚D1-D170H突变体中野生型D1蛋白的产生机理,但在分析D1蛋白中关键残基的定点突变体时必须谨慎,并且必须确认突变不仅在DNA水平,而且在氨基酸水平。作为对照的D1-S169A突变体显示出改良的FTIR光谱,仅显示了突变的D1蛋白。因此可以得出结论,D1-D170H突变体的FTIR光谱实际上反映了野生型PSII的FTIR光谱,而在具有D1-D170H突变的PSII中没有形成Mn4CaO5簇。尽管目前尚不清楚D1-D170H突变体中野生型D1蛋白的产生机理,但在分析D1蛋白中关键残基的定点突变体时必须谨慎,并且必须确认突变不仅在DNA水平,而且在氨基酸水平。











































 京公网安备 11010802027423号
京公网安备 11010802027423号