Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Atomic Structure of the Francisella T6SS Central Spike Reveals a Unique α-Helical Lid and a Putative Cargo.
Structure ( IF 4.4 ) Pub Date : 2019-10-31 , DOI: 10.1016/j.str.2019.10.007 Xue Yang 1 , Daniel L Clemens 2 , Bai-Yu Lee 2 , Yanxiang Cui 3 , Z Hong Zhou 3 , Marcus A Horwitz 4
Structure ( IF 4.4 ) Pub Date : 2019-10-31 , DOI: 10.1016/j.str.2019.10.007 Xue Yang 1 , Daniel L Clemens 2 , Bai-Yu Lee 2 , Yanxiang Cui 3 , Z Hong Zhou 3 , Marcus A Horwitz 4
Affiliation
|
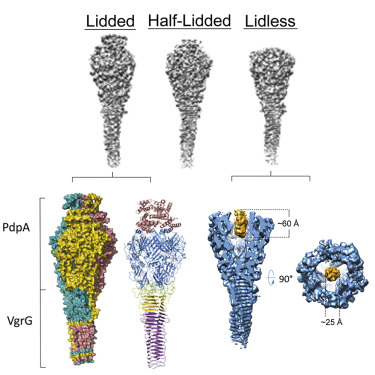
Francisella bacteria rely on a phylogenetically distinct type VI secretion system (T6SS) to escape host phagosomes and cause the fatal disease tularemia, but the structural and molecular mechanisms involved are unknown. Here we report the atomic structure of the Francisella T6SS central spike complex, obtained by cryo-electron microscopy. Our structural and functional studies demonstrate that, unlike the single-protein spike composition of other T6SS subtypes, Francisella T6SS's central spike is formed by two proteins, PdpA and VgrG, akin to T4-bacteriophage gp27 and gp5, respectively, and that PdpA has unique characteristics, including a putative cargo within its cavity and an N-terminal helical lid. Structure-guided mutagenesis demonstrates that the PdpA N-terminal lid and C-terminal spike are essential to Francisella T6SS function. PdpA is thus both an adaptor, connecting VgrG to the tube, and a likely carrier of secreted cargo. These findings are important to understanding Francisella pathogenicity and designing therapeutics to combat tularemia.
中文翻译:

Francisella T6SS Central Spike的原子结构揭示了独特的α螺旋盖和推定的货物。
弗朗西斯菌依靠系统发育上独特的VI型分泌系统(T6SS)逃脱宿主吞噬体并导致致命性疾病Tularemia,但涉及的结构和分子机制尚不清楚。在这里,我们报告了通过低温电子显微镜获得的弗朗西斯菌T6SS中央尖峰复合物的原子结构。我们的结构和功能研究表明,与其他T6SS亚型的单蛋白刺突组成不同,弗朗西斯菌T6SS的中央刺突由分别类似于T4噬菌体gp27和gp5的两种蛋白PdpA和VgrG形成,并且PdpA具有独特的特性,包括在其空腔内的推定货物和一个N端螺旋盖。结构指导的诱变表明,PdpA N端盖和C端突波对于弗朗西斯菌T6SS功能至关重要。因此,PdpA既是将VgrG连接到试管的适配器,又可能是分泌货物的载体。这些发现对于理解弗朗西斯菌的致病性和设计抗tularemia的疗法非常重要。
更新日期:2019-11-01
中文翻译:

Francisella T6SS Central Spike的原子结构揭示了独特的α螺旋盖和推定的货物。
弗朗西斯菌依靠系统发育上独特的VI型分泌系统(T6SS)逃脱宿主吞噬体并导致致命性疾病Tularemia,但涉及的结构和分子机制尚不清楚。在这里,我们报告了通过低温电子显微镜获得的弗朗西斯菌T6SS中央尖峰复合物的原子结构。我们的结构和功能研究表明,与其他T6SS亚型的单蛋白刺突组成不同,弗朗西斯菌T6SS的中央刺突由分别类似于T4噬菌体gp27和gp5的两种蛋白PdpA和VgrG形成,并且PdpA具有独特的特性,包括在其空腔内的推定货物和一个N端螺旋盖。结构指导的诱变表明,PdpA N端盖和C端突波对于弗朗西斯菌T6SS功能至关重要。因此,PdpA既是将VgrG连接到试管的适配器,又可能是分泌货物的载体。这些发现对于理解弗朗西斯菌的致病性和设计抗tularemia的疗法非常重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号