Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phase Separation of Zonula Occludens Proteins Drives Formation of Tight Junctions.
Cell ( IF 45.5 ) Pub Date : 2019-10-31 , DOI: 10.1016/j.cell.2019.10.011 Oliver Beutel 1 , Riccardo Maraspini 1 , Karina Pombo-García 1 , Cécilie Martin-Lemaitre 1 , Alf Honigmann 2
Cell ( IF 45.5 ) Pub Date : 2019-10-31 , DOI: 10.1016/j.cell.2019.10.011 Oliver Beutel 1 , Riccardo Maraspini 1 , Karina Pombo-García 1 , Cécilie Martin-Lemaitre 1 , Alf Honigmann 2
Affiliation
|
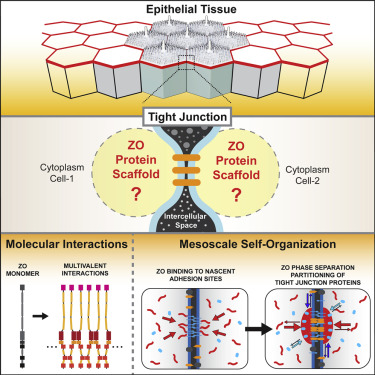
Tight junctions are cell-adhesion complexes that seal tissues and are involved in cell polarity and signaling. Supra-molecular assembly and positioning of tight junctions as continuous networks of adhesion strands are dependent on the membrane-associated scaffolding proteins ZO1 and ZO2. To understand how zona occludens (ZO) proteins organize junction assembly, we performed quantitative cell biology and in vitro reconstitution experiments. We discovered that ZO proteins self-organize membrane-attached compartments via phase separation. We identified the multivalent interactions of the conserved PDZ-SH3-GuK supra-domain as the driver of phase separation. These interactions are regulated by phosphorylation and intra-molecular binding. Formation of condensed ZO protein compartments is sufficient to specifically enrich and localize tight-junction proteins, including adhesion receptors, cytoskeletal adapters, and transcription factors. Our results suggest that an active-phase transition of ZO proteins into a condensed membrane-bound compartment drives claudin polymerization and coalescence of a continuous tight-junction belt.
中文翻译:

Zonula咬合蛋白的相分离驱动紧密连接的形成。
紧密连接是密封组织的细胞粘附复合物,并参与细胞极性和信号传导。作为粘附链的连续网络的超分子组装和紧密连接的定位取决于膜相关的支架蛋白ZO1和ZO2。为了了解透明带遮盖物(ZO)蛋白质如何组织连接组装,我们进行了定量细胞生物学和体外重建实验。我们发现,ZO蛋白通过相分离自组织膜附着区室。我们确定了保守的PDZ-SH3-GuK超域的多价相互作用是相分离的驱动力。这些相互作用通过磷酸化和分子内结合来调节。缩合的ZO蛋白区室的形成足以特异性富集和定位紧密连接的蛋白质,包括粘附受体,细胞骨架衔接子和转录因子。我们的结果表明,ZO蛋白进入凝结的膜结合区室的活性相转变会驱动claudin聚合和连续紧密连接带的聚结。
更新日期:2019-11-09
中文翻译:

Zonula咬合蛋白的相分离驱动紧密连接的形成。
紧密连接是密封组织的细胞粘附复合物,并参与细胞极性和信号传导。作为粘附链的连续网络的超分子组装和紧密连接的定位取决于膜相关的支架蛋白ZO1和ZO2。为了了解透明带遮盖物(ZO)蛋白质如何组织连接组装,我们进行了定量细胞生物学和体外重建实验。我们发现,ZO蛋白通过相分离自组织膜附着区室。我们确定了保守的PDZ-SH3-GuK超域的多价相互作用是相分离的驱动力。这些相互作用通过磷酸化和分子内结合来调节。缩合的ZO蛋白区室的形成足以特异性富集和定位紧密连接的蛋白质,包括粘附受体,细胞骨架衔接子和转录因子。我们的结果表明,ZO蛋白进入凝结的膜结合区室的活性相转变会驱动claudin聚合和连续紧密连接带的聚结。











































 京公网安备 11010802027423号
京公网安备 11010802027423号