当前位置:
X-MOL 学术
›
Lancet Diabetes Endocrinol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Odanacatib for the treatment of postmenopausal osteoporosis: results of the LOFT multicentre, randomised, double-blind, placebo-controlled trial and LOFT Extension study.
The Lancet Diabetes & Endocrinology ( IF 44.5 ) Pub Date : 2019-10-31 , DOI: 10.1016/s2213-8587(19)30346-8 Michael R McClung 1 , Michelle L O'Donoghue 2 , Socrates E Papapoulos 3 , Henry Bone 4 , Bente Langdahl 5 , Kenneth G Saag 6 , Ian R Reid 7 , Douglas P Kiel 8 , Ilaria Cavallari 2 , Marc P Bonaca 2 , Stephen D Wiviott 2 , Tobias de Villiers 9 , Xu Ling 10 , Kurt Lippuner 11 , Toshitaka Nakamura 12 , Jean-Yves Reginster 13 , Jose Adolfo Rodriguez-Portales 14 , Christian Roux 15 , José Zanchetta 16 , Cristiano A F Zerbini 17 , Jeong-Gun Park 2 , KyungAh Im 2 , Abby Cange 2 , Laura T Grip 2 , Norman Heyden 18 , Carolyn DaSilva 18 , Dosinda Cohn 18 , Rachid Massaad 18 , Boyd B Scott 18 , Nadia Verbruggen 18 , Deborah Gurner 18 , Deborah L Miller 18 , Micki L Blair 18 , Adam B Polis 18 , S Aubrey Stoch 18 , Arthur Santora 18 , Antonio Lombardi 18 , Albert T Leung 18 , Keith D Kaufman 18 , Marc S Sabatine 2 ,
The Lancet Diabetes & Endocrinology ( IF 44.5 ) Pub Date : 2019-10-31 , DOI: 10.1016/s2213-8587(19)30346-8 Michael R McClung 1 , Michelle L O'Donoghue 2 , Socrates E Papapoulos 3 , Henry Bone 4 , Bente Langdahl 5 , Kenneth G Saag 6 , Ian R Reid 7 , Douglas P Kiel 8 , Ilaria Cavallari 2 , Marc P Bonaca 2 , Stephen D Wiviott 2 , Tobias de Villiers 9 , Xu Ling 10 , Kurt Lippuner 11 , Toshitaka Nakamura 12 , Jean-Yves Reginster 13 , Jose Adolfo Rodriguez-Portales 14 , Christian Roux 15 , José Zanchetta 16 , Cristiano A F Zerbini 17 , Jeong-Gun Park 2 , KyungAh Im 2 , Abby Cange 2 , Laura T Grip 2 , Norman Heyden 18 , Carolyn DaSilva 18 , Dosinda Cohn 18 , Rachid Massaad 18 , Boyd B Scott 18 , Nadia Verbruggen 18 , Deborah Gurner 18 , Deborah L Miller 18 , Micki L Blair 18 , Adam B Polis 18 , S Aubrey Stoch 18 , Arthur Santora 18 , Antonio Lombardi 18 , Albert T Leung 18 , Keith D Kaufman 18 , Marc S Sabatine 2 ,
Affiliation
|
BACKGROUND
Odanacatib, a cathepsin K inhibitor, reduces bone resorption while maintaining bone formation. Previous work has shown that odanacatib increases bone mineral density in postmenopausal women with low bone mass. We aimed to investigate the efficacy and safety of odanacatib to reduce fracture risk in postmenopausal women with osteoporosis.
METHODS
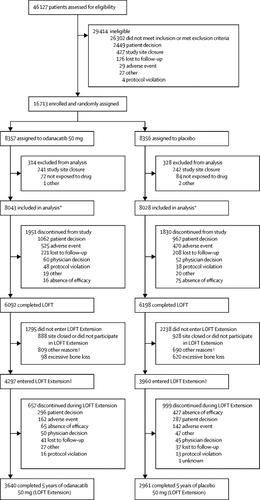
The Long-term Odanacatib Fracture Trial (LOFT) was a multicentre, randomised, double-blind, placebo-controlled, event-driven study at 388 outpatient clinics in 40 countries. Eligible participants were women aged at least 65 years who were postmenopausal for 5 years or more, with a femoral neck or total hip bone mineral density T-score between -2·5 and -4·0 if no previous radiographic vertebral fracture, or between -1·5 and -4·0 with a previous vertebral fracture. Women with a previous hip fracture, more than one vertebral fracture, or a T-score of less than -4·0 at the total hip or femoral neck were not eligible unless they were unable or unwilling to use approved osteoporosis treatment. Participants were randomly assigned (1:1) to either oral odanacatib (50 mg once per week) or matching placebo. Randomisation was done using an interactive voice recognition system after stratification for previous radiographic vertebral fracture, and treatment was masked to study participants, investigators and their staff, and sponsor personnel. If the study completed before 5 years of double-blind treatment, consenting participants could enrol in a double-blind extension study (LOFT Extension), continuing their original treatment assignment for up to 5 years from randomisation. Primary endpoints were incidence of vertebral fractures as assessed using radiographs collected at baseline, 6 and 12 months, yearly, and at final study visit in participants for whom evaluable radiograph images were available at baseline and at least one other timepoint, and hip and non-vertebral fractures adjudicated as being a result of osteoporosis as assessed by clinical history and radiograph. Safety was assessed in participants who received at least one dose of study drug. The adjudicated cardiovascular safety endpoints were a composite of cardiovascular death, myocardial infarction, or stroke, and new-onset atrial fibrillation or flutter. Individual cardiovascular endpoints and death were also assessed. LOFT and LOFT Extension are registered with ClinicalTrials.gov (number NCT00529373) and the European Clinical Trials Database (EudraCT number 2007-002693-66).
FINDINGS
Between Sept 14, 2007, and Nov 17, 2009, we randomly assigned 16 071 evaluable patients to treatment: 8043 to odanacatib and 8028 to placebo. After a median follow-up of 36·5 months (IQR 34·43-40·15) 4297 women assigned to odanacatib and 3960 assigned to placebo enrolled in LOFT Extension (total median follow-up 47·6 months, IQR 35·45-60·06). In LOFT, cumulative incidence of primary outcomes for odanacatib versus placebo were: radiographic vertebral fractures 3·7% (251/6770) versus 7·8% (542/6910), hazard ratio (HR) 0·46, 95% CI 0·40-0·53; hip fractures 0·8% (65/8043) versus 1·6% (125/8028), 0·53, 0·39-0·71; non-vertebral fractures 5·1% (412/8043) versus 6·7% (541/8028), 0·77, 0·68-0·87; all p<0·0001. Combined results from LOFT plus LOFT Extension for cumulative incidence of primary outcomes for odanacatib versus placebo were: radiographic vertebral fractures 4·9% (341/6909) versus 9·6% (675/7011), HR 0·48, 95% CI 0·42-0·55; hip fractures 1·1% (86/8043) versus 2·0% (162/8028), 0·52, 0·40-0·67; non-vertebral fractures 6·4% (512/8043) versus 8·4% (675/8028), 0·74, 0·66-0·83; all p<0·0001. In LOFT, the composite cardiovascular endpoint of cardiovascular death, myocardial infarction, or stroke occurred in 273 (3·4%) of 8043 patients in the odanacatib group versus 245 (3·1%) of 8028 in the placebo group (HR 1·12, 95% CI 0·95-1·34; p=0·18). New-onset atrial fibrillation or flutter occurred in 112 (1·4%) of 8043 patients in the odanacatib group versus 96 (1·2%) of 8028 in the placebo group (HR 1·18, 0·90-1·55; p=0·24). Odanacatib was associated with an increased risk of stroke (1·7% [136/8043] vs 1·3% [104/8028], HR 1·32, 1·02-1·70; p=0·034), but not myocardial infarction (0·7% [60/8043] vs 0·9% [74/8028], HR 0·82, 0·58-1·15; p=0·26). The HR for all-cause mortality was 1·13 (5·0% [401/8043] vs 4·4% [356/8028], 0·98-1·30; p=0·10). When data from LOFT Extension were included, the composite of cardiovascular death, myocardial infarction, or stroke occurred in significantly more patients in the odanacatib group than in the placebo group (401 [5·0%] of 8043 vs 343 [4·3%] of 8028, HR 1·17, 1·02-1·36; p=0·029, as did stroke (2·3% [187/8043] vs 1·7% [137/8028], HR 1·37, 1·10-1·71; p=0·0051).
INTERPRETATION
Odanacatib reduced the risk of fracture, but was associated with an increased risk of cardiovascular events, specifically stroke, in postmenopausal women with osteoporosis. Based on the overall balance between benefit and risk, the study's sponsor decided that they would no longer pursue development of odanacatib for treatment of osteoporosis.
FUNDING
Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Kenilworth, NJ, USA.
中文翻译:

Odanacatib治疗绝经后骨质疏松症:LOFT多中心,随机,双盲,安慰剂对照试验和LOFT延伸研究的结果。
背景技术组织蛋白酶K抑制剂Odanacatib在保持骨骼形成的同时减少了骨骼的吸收。先前的研究表明,odanacatib可提高骨量低的绝经后妇女的骨矿物质密度。我们旨在研究奥达那替尼降低绝经后骨质疏松妇女骨折风险的功效和安全性。方法长期Odanacatib骨折试验(LOFT)是一项在40个国家/地区的388个门诊诊所进行的多中心,随机,双盲,安慰剂对照,事件驱动的研究。符合条件的参与者是至少65岁的女性,绝经后5年或更长时间,股骨颈或总髋骨矿物质密度T分数在-2·-5和-4·0之间(如果先前没有影像学上的椎骨骨折或在此之间)。 -1·5和-4·0伴有先前的椎骨骨折。先前有髋部骨折,多于一个椎骨骨折或在整个髋部或股骨颈的T值低于-4·0的女性,除非她们不能或不愿意使用批准的骨质疏松症治疗,否则不符合资格。参与者被随机分配(1:1)口服奥达卡替尼(每周一次50 mg)或匹配的安慰剂。在对先前的影像学椎骨骨折进行分层后,使用交互式语音识别系统进行随机分组,并对研究参与者,研究人员及其工作人员和赞助人员进行掩盖治疗。如果该研究在5年的双盲治疗之前完成,则同意的参与者可以参加一项双盲扩展研究(LOFT扩展),从随机分组开始最多进行5年的原始治疗。主要终点是在基线,每年6和12个月,每年以及在最终研究访问时使用射线照相所评估的椎体骨折发生率,这些参与者在基线和至少一个其他时间点可获得可评估的射线照相图像,并且髋部和非髋部根据临床病史和X线照片判断,因骨质疏松而判定为椎体骨折。在接受至少一剂研究药物的参与者中评估安全性。裁定的心血管安全终点指标是心血管死亡,心肌梗塞或中风以及新发房颤或扑动的综合结果。还评估了个体心血管终点和死亡。LOFT和LOFT Extension已在ClinicalTrials中注册。gov(编号NCT00529373)和欧洲临床试验数据库(EudraCT编号2007-002693-66)。结果在2007年9月14日至2009年11月17日之间,我们随机分配了16071名可评估患者进行治疗:odanacatib为8043,安慰剂为8028。在中位随访36·5个月(IQR 34·43-40·15)后,接受LOFT扩展的4297名妇女分入odanacatib和3960名接受安慰剂的妇女(总中位随访47·6个月,IQR 35·45 -60·06)。在LOFT中,奥达卡替尼和安慰剂的主要结局累积发生率分别为:椎骨骨折3·7%(251/6770)对7·8%(542/6910),危险比(HR)0·46、95%CI 0 ·40-0·53;髋部骨折0·8%(65/8043)对1·6%(125/8028),0·53、0·39-0·71;非椎骨骨折的发生率分别为5·1%(412/8043)和6·7%(541/8028),0·77、0·68-0·87;全部p <0·0001。奥达那替尼与安慰剂的主要结局累积发生率的从LOFT加上LOFT扩展得出的综合结果为:放射影像性椎体骨折4·9%(341/6909)对9·6%(675/7011),HR 0·48、95%CI 0·42-0·55;髋部骨折1%(1/8%)(86/8043)对2%(0/8%(162/8028)),0·52、0·40-0·67;非椎骨骨折的发生率分别为6·4%(512/8043)和8·4%(675/8028),0·74、0·66-0·83;全部p <0·0001。在LOFT中,odanacatib组的8043名患者中有273名(3·4%)发生了心血管死亡,心肌梗塞或中风的复合心血管终点,而安慰剂组的8028名中有245名(3·1%)(HR 1· 12,95%CI 0·95-1·34; p = 0·18)。odanacatib组8043例患者中有112例发生新发房颤或扑动(1·4%),而安慰剂组8028例中有96例(1·2%)(HR 1·18,0·90-1·55 ; p = 0·24)。Odanacatib与卒中风险增加相关(1·7%[136/8043]比1·3%[104/8028],HR 1·32、1·02-1·70; p = 0·034),但不是心肌梗塞(0·7%[60/8043] vs 0·9%[74/8028],HR 0·82、0·58-1·15; p = 0·26)。全因死亡率的HR为1·13(5·0%[401/8043]对4·4%[356/8028],0·98-1·30; p = 0·10)。包括LOFT Extension的数据时,与安慰剂组相比,与安慰剂组相比,odanacatib组的心血管死亡,心肌梗塞或中风的发生率要高得多(804 [401] [5·0%]与343 [4·3%] []对应于8028,HR 1·17、1·02-1·36; p = 0·029,卒中也是如此(2·3%[187/8043]与1·7%[137/8028],HR 1· 37,1·10-1·71; p = 0·0051)。解释奥达那替尼降低了骨折的风险,但与心血管事件,特别是中风的风险增加有关,在绝经后骨质疏松症妇女中。根据收益与风险之间的总体平衡,研究的发起人决定,他们将不再寻求开发奥达那替尼来治疗骨质疏松症。融资默沙东公司(Merck Sharp&Dohme Corp)是位于美国新泽西州Kenilworth的默克公司(Merck&Co,Inc.)的子公司。
更新日期:2019-11-20
中文翻译:

Odanacatib治疗绝经后骨质疏松症:LOFT多中心,随机,双盲,安慰剂对照试验和LOFT延伸研究的结果。
背景技术组织蛋白酶K抑制剂Odanacatib在保持骨骼形成的同时减少了骨骼的吸收。先前的研究表明,odanacatib可提高骨量低的绝经后妇女的骨矿物质密度。我们旨在研究奥达那替尼降低绝经后骨质疏松妇女骨折风险的功效和安全性。方法长期Odanacatib骨折试验(LOFT)是一项在40个国家/地区的388个门诊诊所进行的多中心,随机,双盲,安慰剂对照,事件驱动的研究。符合条件的参与者是至少65岁的女性,绝经后5年或更长时间,股骨颈或总髋骨矿物质密度T分数在-2·-5和-4·0之间(如果先前没有影像学上的椎骨骨折或在此之间)。 -1·5和-4·0伴有先前的椎骨骨折。先前有髋部骨折,多于一个椎骨骨折或在整个髋部或股骨颈的T值低于-4·0的女性,除非她们不能或不愿意使用批准的骨质疏松症治疗,否则不符合资格。参与者被随机分配(1:1)口服奥达卡替尼(每周一次50 mg)或匹配的安慰剂。在对先前的影像学椎骨骨折进行分层后,使用交互式语音识别系统进行随机分组,并对研究参与者,研究人员及其工作人员和赞助人员进行掩盖治疗。如果该研究在5年的双盲治疗之前完成,则同意的参与者可以参加一项双盲扩展研究(LOFT扩展),从随机分组开始最多进行5年的原始治疗。主要终点是在基线,每年6和12个月,每年以及在最终研究访问时使用射线照相所评估的椎体骨折发生率,这些参与者在基线和至少一个其他时间点可获得可评估的射线照相图像,并且髋部和非髋部根据临床病史和X线照片判断,因骨质疏松而判定为椎体骨折。在接受至少一剂研究药物的参与者中评估安全性。裁定的心血管安全终点指标是心血管死亡,心肌梗塞或中风以及新发房颤或扑动的综合结果。还评估了个体心血管终点和死亡。LOFT和LOFT Extension已在ClinicalTrials中注册。gov(编号NCT00529373)和欧洲临床试验数据库(EudraCT编号2007-002693-66)。结果在2007年9月14日至2009年11月17日之间,我们随机分配了16071名可评估患者进行治疗:odanacatib为8043,安慰剂为8028。在中位随访36·5个月(IQR 34·43-40·15)后,接受LOFT扩展的4297名妇女分入odanacatib和3960名接受安慰剂的妇女(总中位随访47·6个月,IQR 35·45 -60·06)。在LOFT中,奥达卡替尼和安慰剂的主要结局累积发生率分别为:椎骨骨折3·7%(251/6770)对7·8%(542/6910),危险比(HR)0·46、95%CI 0 ·40-0·53;髋部骨折0·8%(65/8043)对1·6%(125/8028),0·53、0·39-0·71;非椎骨骨折的发生率分别为5·1%(412/8043)和6·7%(541/8028),0·77、0·68-0·87;全部p <0·0001。奥达那替尼与安慰剂的主要结局累积发生率的从LOFT加上LOFT扩展得出的综合结果为:放射影像性椎体骨折4·9%(341/6909)对9·6%(675/7011),HR 0·48、95%CI 0·42-0·55;髋部骨折1%(1/8%)(86/8043)对2%(0/8%(162/8028)),0·52、0·40-0·67;非椎骨骨折的发生率分别为6·4%(512/8043)和8·4%(675/8028),0·74、0·66-0·83;全部p <0·0001。在LOFT中,odanacatib组的8043名患者中有273名(3·4%)发生了心血管死亡,心肌梗塞或中风的复合心血管终点,而安慰剂组的8028名中有245名(3·1%)(HR 1· 12,95%CI 0·95-1·34; p = 0·18)。odanacatib组8043例患者中有112例发生新发房颤或扑动(1·4%),而安慰剂组8028例中有96例(1·2%)(HR 1·18,0·90-1·55 ; p = 0·24)。Odanacatib与卒中风险增加相关(1·7%[136/8043]比1·3%[104/8028],HR 1·32、1·02-1·70; p = 0·034),但不是心肌梗塞(0·7%[60/8043] vs 0·9%[74/8028],HR 0·82、0·58-1·15; p = 0·26)。全因死亡率的HR为1·13(5·0%[401/8043]对4·4%[356/8028],0·98-1·30; p = 0·10)。包括LOFT Extension的数据时,与安慰剂组相比,与安慰剂组相比,odanacatib组的心血管死亡,心肌梗塞或中风的发生率要高得多(804 [401] [5·0%]与343 [4·3%] []对应于8028,HR 1·17、1·02-1·36; p = 0·029,卒中也是如此(2·3%[187/8043]与1·7%[137/8028],HR 1· 37,1·10-1·71; p = 0·0051)。解释奥达那替尼降低了骨折的风险,但与心血管事件,特别是中风的风险增加有关,在绝经后骨质疏松症妇女中。根据收益与风险之间的总体平衡,研究的发起人决定,他们将不再寻求开发奥达那替尼来治疗骨质疏松症。融资默沙东公司(Merck Sharp&Dohme Corp)是位于美国新泽西州Kenilworth的默克公司(Merck&Co,Inc.)的子公司。



























 京公网安备 11010802027423号
京公网安备 11010802027423号