当前位置:
X-MOL 学术
›
Drug Test. Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Analysis of tretoquinol and its metabolites in human urine by liquid chromatography-tandem mass spectrometry.
Drug Testing and Analysis ( IF 2.6 ) Pub Date : 2019-12-29 , DOI: 10.1002/dta.2714 Masato Okano 1 , Asami Miyamoto 1 , Mitsuhiko Sato 1 , Shinji Kageyama 1
Drug Testing and Analysis ( IF 2.6 ) Pub Date : 2019-12-29 , DOI: 10.1002/dta.2714 Masato Okano 1 , Asami Miyamoto 1 , Mitsuhiko Sato 1 , Shinji Kageyama 1
Affiliation
|
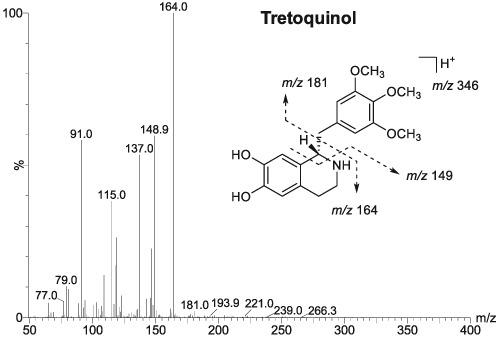
Tretoquinol (trimetoquinol), a β2‐agonist, has been explicitly listed on the World Anti‐Doping Agency Prohibited List 2019 since January 2019; however, it has been distributed as an antiasthmatic on the medical market. This study aimed to develop a liquid chromatography–tandem mass spectrometric method for the quantification of tretoquinol (free form plus glucuronide) in human urine for doping control purposes. An excretion study (n = 6) of tretoquinol hydrochloride hydrate (6 mg) was performed, and urine samples were collected prior to oral administration and during the first 48 h, along with spot urine samples at 7 and 14 days after administration. All the urine samples were analysed using the developed method. The limit of detection for the developed method was 0.03 ng/mL. The inter‐day precision for the target analyte was excellent (2.7% to 9.2%), and the inter‐day accuracy of target analyte was −0.6% to −3.6%. In all subjects, tretoquinol (free form plus glucuronide conjugate) was identified up to 48 h after administration. The maximum concentrations were in the range of 12.4–78.8 ng/mL and the mean concentration was 55.3 ng/mL. The metabolites O‐methylated tretoquinol, tretoquinol sulphate and O‐methylated tretoquinol sulphate could be also identified in human urine after administration. The longest‐lasting urinary metabolite of tretoquinol currently known, O‐methylated tretoquinol, is also likely to be a useful marker in doping controls.
中文翻译:

液相色谱-串联质谱法分析人尿中的对苯二酚及其代谢产物。
自2019年1月起,β2-激动剂Tretoquinol(trimetoquinol)已明确列入《 2019年世界反兴奋剂机构禁用清单》; 然而,它已作为一种平喘药在医疗市场上分发。这项研究旨在开发一种液相色谱-串联质谱法,用于定量控制尿液中人体内的对苯二酚(游离形式加葡萄糖醛酸)。排泄研究(n= 6)进行盐酸对苯二酚水合物(6mg)的测定,并在口服给药之前和最初的48小时内收集尿液样品,并在给药后7和14天收集点尿液样品。使用开发的方法分析所有尿液样本。所开发方法的检出限为0.03 ng / mL。目标分析物的日间准确度极佳(2.7%至9.2%),目标分析物的日间准确度为-0.6%至-3.6%。在所有受试者中,在给药后48小时内均确认了tretoquinol(游离形式加葡萄糖醛酸偶联物)。最大浓度为12.4-78.8 ng / mL,平均浓度为55.3 ng / mL。代谢物O-甲基化对苯二酚,硫酸对苯二酚和O-服用后在人尿中也可以鉴定出甲基化的对苯二酚硫酸盐。目前已知的最长寿命的对苯二酚尿液代谢物O-甲基化的对苯二酚也可能是兴奋剂控制中的有用标志物。
更新日期:2019-12-29
中文翻译:

液相色谱-串联质谱法分析人尿中的对苯二酚及其代谢产物。
自2019年1月起,β2-激动剂Tretoquinol(trimetoquinol)已明确列入《 2019年世界反兴奋剂机构禁用清单》; 然而,它已作为一种平喘药在医疗市场上分发。这项研究旨在开发一种液相色谱-串联质谱法,用于定量控制尿液中人体内的对苯二酚(游离形式加葡萄糖醛酸)。排泄研究(n= 6)进行盐酸对苯二酚水合物(6mg)的测定,并在口服给药之前和最初的48小时内收集尿液样品,并在给药后7和14天收集点尿液样品。使用开发的方法分析所有尿液样本。所开发方法的检出限为0.03 ng / mL。目标分析物的日间准确度极佳(2.7%至9.2%),目标分析物的日间准确度为-0.6%至-3.6%。在所有受试者中,在给药后48小时内均确认了tretoquinol(游离形式加葡萄糖醛酸偶联物)。最大浓度为12.4-78.8 ng / mL,平均浓度为55.3 ng / mL。代谢物O-甲基化对苯二酚,硫酸对苯二酚和O-服用后在人尿中也可以鉴定出甲基化的对苯二酚硫酸盐。目前已知的最长寿命的对苯二酚尿液代谢物O-甲基化的对苯二酚也可能是兴奋剂控制中的有用标志物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号