Joule ( IF 39.8 ) Pub Date : 2019-10-30 , DOI: 10.1016/j.joule.2019.10.001 Jingyuan Liu , Xiangwen Gao , Gareth O. Hartley , Gregory J. Rees , Chen Gong , Felix H. Richter , Jürgen Janek , Yongyao Xia , Alex W. Robertson , Lee R. Johnson , Peter G. Bruce
|
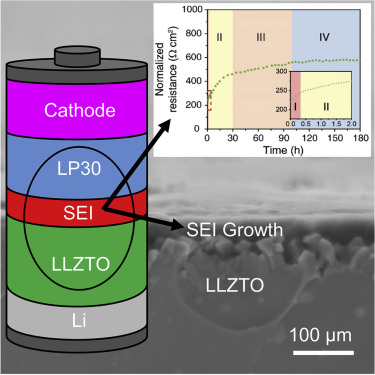
An advantageous solid electrolyte/liquid electrolyte interface is crucial for the implementation of a protected lithium anode in liquid electrolyte cells. Li6.5La3Zr1.5Ta0.5O12 (LLZTO) garnet electrolytes are among the few solid electrolytes that are stable in contact with lithium metal. We show LLZTO is unstable in contact with the organic carbonate-based Li+ liquid electrolyte used in conventional Li-ion cells. The interfacial resistance between LLZTO and LiPF6 in (CH2O)2CO: OC(OCH3)2 (1:1 v/v) increases with time due to the growth of a lithium-ion-conducting solid electrolyte interphase (SEI) at the surface of the ceramic electrolyte. The interphase is composed of Li2CO3, LiF, Li2O, and organic carbonates. Even at a rate of 5 mA cm−2, a 3 V potential drop occurs across the LLZTO/liquid electrolyte interface. A practical LLZTO membrane (thickness ∼10 μm), in contact with a lithium anode, gives a potential loss of ∼16 mV, less than 1% of the resistance of the SEI.
中文翻译:

Li 6.5 La 3 Zr 1.5 Ta 0.5 O 12与液体电解质的界面
有利的固体电解质/液体电解质界面对于在液体电解质电池中实施受保护的锂阳极至关重要。Li 6.5 La 3 Zr 1.5 Ta 0.5 O 12(LLZTO)石榴石电解质是少数与锂金属接触稳定的固体电解质。我们显示LLZTO与常规锂离子电池中使用的基于有机碳酸盐的Li +液体电解质接触时不稳定。LLZTO和LiPF 6在(CH 2 O)2 CO:OC(OCH 3)2中的界面电阻(1:1 v / v)随着时间的增加而增加,这是由于在陶瓷电解质表面上传导锂离子的固体电解质中间相(SEI)的增长。该中间相由Li 2 CO 3,LiF,Li 2 O和有机碳酸盐组成。即使以5 mA cm -2的速率,在LLZTO /液体电解质界面上也会出现3 V的电位降。实用的LLZTO膜(厚度约10μm)与锂阳极接触,会产生约16 mV的电势损失,不到SEI电阻的1%。



























 京公网安备 11010802027423号
京公网安备 11010802027423号